Ab Initio Quantum Chemistry: Thermochemistry and Kinetics
... molecule like H2 or CH4 in vacuo. With energies, • Most simply, the goal of electronic structure w e can optimize calculations is energy. molecular structure Energy E [from • However, usually we want x EH ] energy of an optimized x structure and the energy’s variation with structure. x Position r ...
... molecule like H2 or CH4 in vacuo. With energies, • Most simply, the goal of electronic structure w e can optimize calculations is energy. molecular structure Energy E [from • However, usually we want x EH ] energy of an optimized x structure and the energy’s variation with structure. x Position r ...
Chemistry General v. 2016
... Differentiate between physical and chemical properties and predict how combinations of substances can result in physical and/or chemical changes. Explain the difference between endothermic and exothermic reactions and processes. Describe the relationship between average kinetic molecular energy, tem ...
... Differentiate between physical and chemical properties and predict how combinations of substances can result in physical and/or chemical changes. Explain the difference between endothermic and exothermic reactions and processes. Describe the relationship between average kinetic molecular energy, tem ...
State Standard - SchoolNotes.com
... C-3.3 Explain how the types of intermolecular forces present in a compound affect the physical properties of compounds (including polarity and molecular shape). C-3.4 Explain the unique bonding characteristics of carbon that have resulted in the formation of a large variety of organic structures. C- ...
... C-3.3 Explain how the types of intermolecular forces present in a compound affect the physical properties of compounds (including polarity and molecular shape). C-3.4 Explain the unique bonding characteristics of carbon that have resulted in the formation of a large variety of organic structures. C- ...
Chemical Reaction
... breaks down to form two or more simpler substances. (synonyms: corrode, decay, breakdown) • Single Displacement: Sometimes, an element replaces another element that is a part of a compound. This type of reaction is called a single-displacement reaction. (synonyms: move, shift, rearrange) • Double Di ...
... breaks down to form two or more simpler substances. (synonyms: corrode, decay, breakdown) • Single Displacement: Sometimes, an element replaces another element that is a part of a compound. This type of reaction is called a single-displacement reaction. (synonyms: move, shift, rearrange) • Double Di ...
Conducting Strip by Conjugate Functions
... For y a, V (0, y) ∼ Φ0 y/a, which corresponds to a constant field; in this limit the strip may be approximated by a charged plane. For y a, V (0, y) ∼ Φ0 ln(2y/a),† which corresponds to a charged wire; in this limit the strip may be approximated by a line. Figure 2 shows V (0, y)/Φ0 as a functio ...
... For y a, V (0, y) ∼ Φ0 y/a, which corresponds to a constant field; in this limit the strip may be approximated by a charged plane. For y a, V (0, y) ∼ Φ0 ln(2y/a),† which corresponds to a charged wire; in this limit the strip may be approximated by a line. Figure 2 shows V (0, y)/Φ0 as a functio ...
Electric Potential
... surfaces. In both diagrams the potential difference between adjacent equipotentials is the same. Which of these two could represent the field of a point charge? (A) a (B) b (C) neither a or b Field gets stronger at higher r in (b) ...
... surfaces. In both diagrams the potential difference between adjacent equipotentials is the same. Which of these two could represent the field of a point charge? (A) a (B) b (C) neither a or b Field gets stronger at higher r in (b) ...
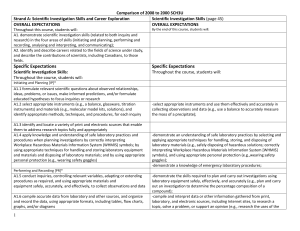
Comparison of 2008 to 2000 SCH3U_ud
... compounds can be released into the environment, including cadmium, arsenic, sulphur dioxide, and mercury, all of which can endanger the health and safety of local populations. Sample questions: What are some chemical reactions used in the manufacture of paper? How might the reactants or products of ...
... compounds can be released into the environment, including cadmium, arsenic, sulphur dioxide, and mercury, all of which can endanger the health and safety of local populations. Sample questions: What are some chemical reactions used in the manufacture of paper? How might the reactants or products of ...
Section 6: Electromagnetic Radiation
... Maxwell’s equations consist of a set of coupled first-order partial differential equations relating the various components of electric and magnetic fields. They can be solved as they stand in simple situations. But it is often convenient to introduce potentials, obtaining a smaller number of second- ...
... Maxwell’s equations consist of a set of coupled first-order partial differential equations relating the various components of electric and magnetic fields. They can be solved as they stand in simple situations. But it is often convenient to introduce potentials, obtaining a smaller number of second- ...
Structure and stability of CaH2 surfaces
... energy; SCaH2 is entropy; and VCaH2 is volume; T and p are applied temperature and pressure of the system. Throughout the present work, we approximate the Gibbs free energy to the total energy obtained from ab initio calculation, i.e., ...
... energy; SCaH2 is entropy; and VCaH2 is volume; T and p are applied temperature and pressure of the system. Throughout the present work, we approximate the Gibbs free energy to the total energy obtained from ab initio calculation, i.e., ...
Chemistry - School District of Springfield Township
... • The Periodic Table evolved over time as scientists discovered more useful ways to compare and organize the elements. o Elements with similar properties have been placed into groups. o The physical and chemical properties of the elements repeat in a regular pattern when they are arranged in order o ...
... • The Periodic Table evolved over time as scientists discovered more useful ways to compare and organize the elements. o Elements with similar properties have been placed into groups. o The physical and chemical properties of the elements repeat in a regular pattern when they are arranged in order o ...
LESSON 23: Exploding Bags
... are called products. In this experiment, students work with common household vinegar and baking soda, which forms a basic solution when dissolved in water. Adding baking soda to vinegar starts a chemical reaction that produces sodium acetate and carbonic acid. The carbonic acid is unstable and insta ...
... are called products. In this experiment, students work with common household vinegar and baking soda, which forms a basic solution when dissolved in water. Adding baking soda to vinegar starts a chemical reaction that produces sodium acetate and carbonic acid. The carbonic acid is unstable and insta ...
Exercises to the Textbook “Physical Chemistry from
... a) Calculate at first the increase ΔV in volume when 20 g of carbonate are used. We imagine that the produced carbon dioxide gas drives back the air over it. Hint: Remember that one mole of any gas, be it pure or mixed, has a volume V of approx. 24.8 L at standard conditions (25 °C, 100 kPa). Theref ...
... a) Calculate at first the increase ΔV in volume when 20 g of carbonate are used. We imagine that the produced carbon dioxide gas drives back the air over it. Hint: Remember that one mole of any gas, be it pure or mixed, has a volume V of approx. 24.8 L at standard conditions (25 °C, 100 kPa). Theref ...
Chapter 19 Chemical Thermodynamics
... • Molecules exhibit several types of motion: – Translational: Movement of the entire molecule from one place to another. – Vibrational: Periodic motion of atoms within a molecule. – Rotational: Rotation of the molecule on about an axis or ...
... • Molecules exhibit several types of motion: – Translational: Movement of the entire molecule from one place to another. – Vibrational: Periodic motion of atoms within a molecule. – Rotational: Rotation of the molecule on about an axis or ...
Chapter 19 Chemical Thermodynamics
... • Molecules exhibit several types of motion: – Translational: Movement of the entire molecule from one place to another. – Vibrational: Periodic motion of atoms within a molecule. – Rotational: Rotation of the molecule on about an axis or ...
... • Molecules exhibit several types of motion: – Translational: Movement of the entire molecule from one place to another. – Vibrational: Periodic motion of atoms within a molecule. – Rotational: Rotation of the molecule on about an axis or ...
Chapter Five
... The molar mass of a substance (be it an element or compound) is the mass of one mole of that substance Molar Mass The mass of molecules can be calculated by adding up the atomic weights of the individual atoms making up the molecule. For example, suppose we wanted to know the molar mass of CO2 ...
... The molar mass of a substance (be it an element or compound) is the mass of one mole of that substance Molar Mass The mass of molecules can be calculated by adding up the atomic weights of the individual atoms making up the molecule. For example, suppose we wanted to know the molar mass of CO2 ...