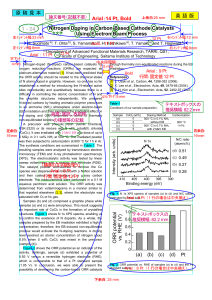
Eötvös Loránd Science University Faculty of Sciences Department of
... exams during the semester. They also get a microproject and are supposed to write an essay- or publication-like report on the results obtained. Add/Drop courses (Week 1); Subscription to courses (1st week); Lecture 1 Information ont he curriculum and the conditions to get a final grade. Reaction kin ...
... exams during the semester. They also get a microproject and are supposed to write an essay- or publication-like report on the results obtained. Add/Drop courses (Week 1); Subscription to courses (1st week); Lecture 1 Information ont he curriculum and the conditions to get a final grade. Reaction kin ...
chapter 18 (moore) - Salisbury University
... As shown previously, H2 and O2 combine spontaneously to form water (exothermic) BUT … … liquid water vaporizes spontaneously at room temperature; an endothermic process. Conclusion: enthalpy alone is not a sufficient criterion for prediction of spontaneity. Entropy (S) and Entropy Change (ΔS) Entrop ...
... As shown previously, H2 and O2 combine spontaneously to form water (exothermic) BUT … … liquid water vaporizes spontaneously at room temperature; an endothermic process. Conclusion: enthalpy alone is not a sufficient criterion for prediction of spontaneity. Entropy (S) and Entropy Change (ΔS) Entrop ...
Chapter One Chemistry
... but are bonds. not chemically combined. how matter changes. An element is a apure that cannot be or broken A puresubstance substance made In compound chemistry, aissubstance is a single kindofoftwo matter down into any other substances by chemical or In a is heterogeneous mixture, you see more eleme ...
... but are bonds. not chemically combined. how matter changes. An element is a apure that cannot be or broken A puresubstance substance made In compound chemistry, aissubstance is a single kindofoftwo matter down into any other substances by chemical or In a is heterogeneous mixture, you see more eleme ...
PCSD General Chemistry Pacing Guide
... Determine the number of significant figures in a calculated result Use dimensional analysis to solve various types of problems Learn the three temperature scales and convert from one to another Minimum Lab Experience ...
... Determine the number of significant figures in a calculated result Use dimensional analysis to solve various types of problems Learn the three temperature scales and convert from one to another Minimum Lab Experience ...
Electronic structure and reactivity analysis of some TTF
... The synthesis and characterization of new heterocyclic systems based on chalcogens (oxygen, sulfur, selenium and tellurium) has been one of the central objectives in contemporary organic chemistry. Several interesting systems have been synthesized and characterized. Especially, sulfur-based heterocy ...
... The synthesis and characterization of new heterocyclic systems based on chalcogens (oxygen, sulfur, selenium and tellurium) has been one of the central objectives in contemporary organic chemistry. Several interesting systems have been synthesized and characterized. Especially, sulfur-based heterocy ...
Name __KEY____________ Per. ______ Polarity and
... conserved, meaning stays the same, during a chemical reaction are [circle 2] (number and type of atoms/ number of molecules/ mass/ volume/ temperature). There are 4 basic types of chemical reactions which are [list them]: 1. ____combination____ 2. ____ decomposition____ 3. ____ single substitution__ ...
... conserved, meaning stays the same, during a chemical reaction are [circle 2] (number and type of atoms/ number of molecules/ mass/ volume/ temperature). There are 4 basic types of chemical reactions which are [list them]: 1. ____combination____ 2. ____ decomposition____ 3. ____ single substitution__ ...
SOLVING L-L EXTRACTION PROBLEMS WITH EXCEL
... iquid-liquid (L-L) extraction, sometimes called solvent extraction, is the separation of the components of a liquid solution by contact with another insoluble liquid. One method used to solve this problem is the equilibrium stage concept.[1] Most L-L extraction processes concern only one component t ...
... iquid-liquid (L-L) extraction, sometimes called solvent extraction, is the separation of the components of a liquid solution by contact with another insoluble liquid. One method used to solve this problem is the equilibrium stage concept.[1] Most L-L extraction processes concern only one component t ...