Chemistry: Matter and Change
... • A change that alters a substance without changing its composition is known as a physical change. • A phase change is a transition of matter from one state to another. • Boiling, freezing, melting, and condensing all describe phase changes in chemistry. ...
... • A change that alters a substance without changing its composition is known as a physical change. • A phase change is a transition of matter from one state to another. • Boiling, freezing, melting, and condensing all describe phase changes in chemistry. ...
Section 6.3 Balancing Chemical Equations
... when a salt dissolves, its ions separate. 2. Consider the various solids that could form. To do this, simply exchange the anions of the added salts. 3. Use the solubility rules to decide whether a solid forms and, if so, to predict the identity of the solid. Return to TOC ...
... when a salt dissolves, its ions separate. 2. Consider the various solids that could form. To do this, simply exchange the anions of the added salts. 3. Use the solubility rules to decide whether a solid forms and, if so, to predict the identity of the solid. Return to TOC ...
Writing Chemical Formulas
... compound. Use the oxidation number (without the plus or minus) for each half as the subscript for the other half. Do not write a subscript of 1. Reduce the subscripts, if needed. After doing this, be sure the subscripts will not reduce. If both subscripts are divisible by the same number, they must ...
... compound. Use the oxidation number (without the plus or minus) for each half as the subscript for the other half. Do not write a subscript of 1. Reduce the subscripts, if needed. After doing this, be sure the subscripts will not reduce. If both subscripts are divisible by the same number, they must ...
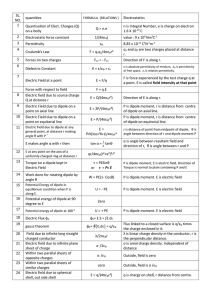
Thermo applications
... if the enthalpies of all the chemical species are calculated relative to the same reference state, namely that of the elements in their standard states at 25°C and 1 atm. By choosing the elemental reference state, the difference in enthalpy can be calculated without having to take into account any o ...
... if the enthalpies of all the chemical species are calculated relative to the same reference state, namely that of the elements in their standard states at 25°C and 1 atm. By choosing the elemental reference state, the difference in enthalpy can be calculated without having to take into account any o ...
NEET 2017 Syllabus PDF Here
... atomization, sublimation, phase transition, ionization, solution and dilution. • Introduction of entropy as state function, Second law of thermodynamics, Gibbs energy change for spontaneous and non-spontaneous process, criteria for equilibrium and spontaneity. • Third law of thermodynamics- Brief in ...
... atomization, sublimation, phase transition, ionization, solution and dilution. • Introduction of entropy as state function, Second law of thermodynamics, Gibbs energy change for spontaneous and non-spontaneous process, criteria for equilibrium and spontaneity. • Third law of thermodynamics- Brief in ...
NEET2017-Entrance Exam Syllabus
... sublimation, phase transition, ionization, solution and dilution. Introduction of entropy as state function, Second law of thermodynamics, Gibbs energy change for spontaneous and non-spontaneous process, criteria for equilibrium and spontaneity. ...
... sublimation, phase transition, ionization, solution and dilution. Introduction of entropy as state function, Second law of thermodynamics, Gibbs energy change for spontaneous and non-spontaneous process, criteria for equilibrium and spontaneity. ...
Errors in Chemical Sensor Measurements
... An analytical chemist performing tests of chemical sensors should be very conscious about possible sources of errors influencing the measurements. Some of them have chemical origin and as quite well predicted and recognised can be determined with the help of analytical methods (e.g. determination of ...
... An analytical chemist performing tests of chemical sensors should be very conscious about possible sources of errors influencing the measurements. Some of them have chemical origin and as quite well predicted and recognised can be determined with the help of analytical methods (e.g. determination of ...
Chapter 6 Chemical Reactions: An Introduction
... • Shorthand way of describing a reaction • Provides information about the reaction: – Formulas of reactants and products – States of reactants and products – Relative numbers of reactant and product molecules that are required – Can be used to determine weights of reactants used and of products that ...
... • Shorthand way of describing a reaction • Provides information about the reaction: – Formulas of reactants and products – States of reactants and products – Relative numbers of reactant and product molecules that are required – Can be used to determine weights of reactants used and of products that ...
Chapter 4 Nomenclature and Chemical Equations
... Depending on the type of bonds present in a compound, different rules are applied to its naming. ...
... Depending on the type of bonds present in a compound, different rules are applied to its naming. ...
CHEMICAL ENGINEERING CHE
... concurrently) or designated score on Mathematics placement test. Not open to students with credit in CEM 142 or CEM 181H or LBS 172. Atomic and molecular structure; ionic and molecular bonding models; periodic trends; chemical reactivity by periodic group; nomenclature, structure, bonding and reacti ...
... concurrently) or designated score on Mathematics placement test. Not open to students with credit in CEM 142 or CEM 181H or LBS 172. Atomic and molecular structure; ionic and molecular bonding models; periodic trends; chemical reactivity by periodic group; nomenclature, structure, bonding and reacti ...
L2004-01A
... LONDON (Reuters) - Robot fish developed by British scientists are to be released into the sea off north Spain to detect pollution. If next year's trial of the first five robotic fish in the northern Spanish port of Gijon is successful, the team hopes they will be used in rivers, lakes and seas acros ...
... LONDON (Reuters) - Robot fish developed by British scientists are to be released into the sea off north Spain to detect pollution. If next year's trial of the first five robotic fish in the northern Spanish port of Gijon is successful, the team hopes they will be used in rivers, lakes and seas acros ...
Lecture Slides - School of Chemical Sciences
... “Simple systems”: Macroscopically homogeneous, isotropic, uncharged, large enough that surface effects can be neglected, not acted upon by electric, magnetic, or gravitational fields. Only those few particular combinations of atomic coordinates that are essentially time-independent are macroscopic ...
... “Simple systems”: Macroscopically homogeneous, isotropic, uncharged, large enough that surface effects can be neglected, not acted upon by electric, magnetic, or gravitational fields. Only those few particular combinations of atomic coordinates that are essentially time-independent are macroscopic ...
Role of Chemistry in Everyday Life
... we eat have to do with chemistry. They consist of organic compounds like carbohydrates starch and sugar, protein, and lipids. Other nutrients like vitamins and minerals and water are all important chemical compounds. The process of respiration removes oxygen from the environment while adding carbon ...
... we eat have to do with chemistry. They consist of organic compounds like carbohydrates starch and sugar, protein, and lipids. Other nutrients like vitamins and minerals and water are all important chemical compounds. The process of respiration removes oxygen from the environment while adding carbon ...