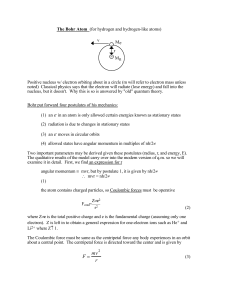
c - Greer Middle College
... Concluded - light has properties of both waves and particles “wave-particle duality” Photon - particle of light that carries a quantum of energy The energy of a photon is proportional to its frequency. E: energy (J, joules) h: Planck’s constant (6.6262 10-34 J·s) ...
... Concluded - light has properties of both waves and particles “wave-particle duality” Photon - particle of light that carries a quantum of energy The energy of a photon is proportional to its frequency. E: energy (J, joules) h: Planck’s constant (6.6262 10-34 J·s) ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI
... State any two postulates of quantum mechanics. Prove that the square of the angular momentum commutes with its components. What is meant by degeneracy of an energy level? What is an orthonormal basis? With an example explain simultaneous eigenfunctions. If A and B are two operators, then show that [ ...
... State any two postulates of quantum mechanics. Prove that the square of the angular momentum commutes with its components. What is meant by degeneracy of an energy level? What is an orthonormal basis? With an example explain simultaneous eigenfunctions. If A and B are two operators, then show that [ ...
IntroQuantumNuclearp..
... • Theoretical models evolved as experimental observations provided more insight, especially the strange quantum phenomena... ...
... • Theoretical models evolved as experimental observations provided more insight, especially the strange quantum phenomena... ...
PHY4605–Introduction to Quantum Mechanics II Spring 1997 Problem Set 4 Jan. 31, 2005
... PHY4605–Introduction to Quantum Mechanics II Spring 1997 Problem Set 4 Jan. 31, 2005 Due: Feb. 7, 2005 Reading: Griffiths, Ch. 6 1. Finite extent of proton. The binding energy and ground state of an electron in an H-atom are normally obtained under the assumption that the proton is a fixed point cha ...
... PHY4605–Introduction to Quantum Mechanics II Spring 1997 Problem Set 4 Jan. 31, 2005 Due: Feb. 7, 2005 Reading: Griffiths, Ch. 6 1. Finite extent of proton. The binding energy and ground state of an electron in an H-atom are normally obtained under the assumption that the proton is a fixed point cha ...
Take silver atoms with an electron that has a moment of µz = −g e(e
... If the electrons have two different kinds of spin directions, atoms with those electrons should have different total spins and then they should respond differently to magnetic fields. In the Stern-Gerlach experiment they send these atoms in the x direction between magnets one above the other, where ...
... If the electrons have two different kinds of spin directions, atoms with those electrons should have different total spins and then they should respond differently to magnetic fields. In the Stern-Gerlach experiment they send these atoms in the x direction between magnets one above the other, where ...
Final Exam 2004
... two atoms. For large R, the dipole-dipole interaction can be considered as a small perturbation. Show that the energy of the dipole-dipole interaction of the two atoms in their ground states is zero in the first order of the perturbation theory. [Hint: Since the ground state is nondegenerate, you ca ...
... two atoms. For large R, the dipole-dipole interaction can be considered as a small perturbation. Show that the energy of the dipole-dipole interaction of the two atoms in their ground states is zero in the first order of the perturbation theory. [Hint: Since the ground state is nondegenerate, you ca ...
Ch 7 Lecture Notes
... Niels Bohr (1885-1962) A Danish Scientist - In 1913 he proposed a new model of the atom that attempted to better explain atomic line spectra and disproved J.J. Thompson’s “Plum Pudding” model. - Electrons move in circular ____________ around the nucleus. - The closer the orbit to the nucleus, the lo ...
... Niels Bohr (1885-1962) A Danish Scientist - In 1913 he proposed a new model of the atom that attempted to better explain atomic line spectra and disproved J.J. Thompson’s “Plum Pudding” model. - Electrons move in circular ____________ around the nucleus. - The closer the orbit to the nucleus, the lo ...
Bohr Model of the Atom
... The Danish physicist Niels Bohr, who first presented this model of the atom, based it on 3 fundamental postulates. (1) Electrons move around the nucleus in circular non-radiating orbits - called “stationary states”. However, they are not at rest! (2) An atom only emits or absorbs electromagnetic rad ...
... The Danish physicist Niels Bohr, who first presented this model of the atom, based it on 3 fundamental postulates. (1) Electrons move around the nucleus in circular non-radiating orbits - called “stationary states”. However, they are not at rest! (2) An atom only emits or absorbs electromagnetic rad ...
The Quantum Theory of Atoms and Molecules
... Same as the Rydberg formula Bohr’s theory is in agreement with experimentally observed spectra. Combining de Broglie’s relation with Bohr condition shows that the circumference of the orbit of radius r must be an integer number of wavelengths, . ...
... Same as the Rydberg formula Bohr’s theory is in agreement with experimentally observed spectra. Combining de Broglie’s relation with Bohr condition shows that the circumference of the orbit of radius r must be an integer number of wavelengths, . ...
NIELS BOHR power point22222
... This description of atomic structure is known as the Bohr atomic model. ...
... This description of atomic structure is known as the Bohr atomic model. ...
CHM1045 General Chemistry and Qualitative Analysis
... 2. Differentiating the structure of the periodic table in terms of groups and periods; classifying elements as metals, nonmetals, or metalloids; and/or according to their group's name as: noble gases, alkali metals, alkaline earth metals, or halogens. Distinguishing among maingroup elements, tra ...
... 2. Differentiating the structure of the periodic table in terms of groups and periods; classifying elements as metals, nonmetals, or metalloids; and/or according to their group's name as: noble gases, alkali metals, alkaline earth metals, or halogens. Distinguishing among maingroup elements, tra ...
Atomic and Molecular Physics for Physicists Ben-Gurion University of the Negev
... One of the sides (a process called 2-photon interference), and never one there and one there. (note that the two photons come from the same source And thus have the same phase). Incoming single photon Do they have to arrive at the mirror at the same time? ...
... One of the sides (a process called 2-photon interference), and never one there and one there. (note that the two photons come from the same source And thus have the same phase). Incoming single photon Do they have to arrive at the mirror at the same time? ...
The Hydrogen Atom
... equal to the circumference of its ground state (the innermost orbit with n=1). If we consider the vibrations of a wire loop, we find that their wavelengths always fit a whole number of times into the loop’s circumference. An electron can circle a nucleus only in orbits that contain an integral numbe ...
... equal to the circumference of its ground state (the innermost orbit with n=1). If we consider the vibrations of a wire loop, we find that their wavelengths always fit a whole number of times into the loop’s circumference. An electron can circle a nucleus only in orbits that contain an integral numbe ...