Quantum Numbers
... f) Label each of the orbital pictures found in question 78 (page 329)with the appropriate letter: g) When n=5, the possible values of l are ______. h) The maximum number of orbitals that can be assigned to the n=4 shell is ____. ...
... f) Label each of the orbital pictures found in question 78 (page 329)with the appropriate letter: g) When n=5, the possible values of l are ______. h) The maximum number of orbitals that can be assigned to the n=4 shell is ____. ...
Chapter 7
... the orientation in space of the orbital. The possible values of this quantum number are –l 0 +l. When l is not zero, the magnetic q.n. has more than one value. These multiple values produce degenerative orbitals – orbitals of equal energy. ...
... the orientation in space of the orbital. The possible values of this quantum number are –l 0 +l. When l is not zero, the magnetic q.n. has more than one value. These multiple values produce degenerative orbitals – orbitals of equal energy. ...
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
... quantum number and i specifies the name of the assumed-distinguishable electron. Since s = 1/2 for all electrons, we can use an abbreviated notation for spin-orbitals: lwhere corresponds to ms = +1/2 and to ms = –1/2. The two-electron basis states are denoted ...
... quantum number and i specifies the name of the assumed-distinguishable electron. Since s = 1/2 for all electrons, we can use an abbreviated notation for spin-orbitals: lwhere corresponds to ms = +1/2 and to ms = –1/2. The two-electron basis states are denoted ...
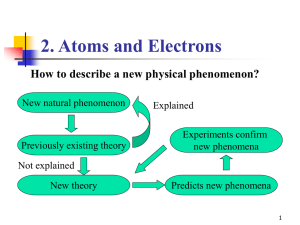
The Atom and Its Properties
... Albert Einstein (18791955) proposed that while a beam of light had wavelike characteristics, it also can be thought of as a stream of tiny particles (or bundles of energy) called photons • Each photon carries a quantum of energy ...
... Albert Einstein (18791955) proposed that while a beam of light had wavelike characteristics, it also can be thought of as a stream of tiny particles (or bundles of energy) called photons • Each photon carries a quantum of energy ...
The Second Law of Thermodynamics
... The physical and chemical properties of elements is determined by the atomic structure. The atomic structure is, in turn, determined by the electrons and which shells, subshells and orbitals they reside in. The rules of placing electrons within shells is known as the Aufbau principle. As protons are ...
... The physical and chemical properties of elements is determined by the atomic structure. The atomic structure is, in turn, determined by the electrons and which shells, subshells and orbitals they reside in. The rules of placing electrons within shells is known as the Aufbau principle. As protons are ...
course materials
... This course considers the theoretical treatment of the geometrical and electronic structure of solids and surfaces and of molecule-surface interactions. For molecules a much-used approximation is molecular orbital (MO) theory, similarly, for crystalline solids and surfaces a widely used approximatio ...
... This course considers the theoretical treatment of the geometrical and electronic structure of solids and surfaces and of molecule-surface interactions. For molecules a much-used approximation is molecular orbital (MO) theory, similarly, for crystalline solids and surfaces a widely used approximatio ...
Chapter 5
... which the elements are separated into groups based on a set of repeating properties. • The periodic table allows you to easily compare the properties of one element to another. • Each horizontal row is called a period. • Each vertical column is called a group, or family. – Elements in a group typica ...
... which the elements are separated into groups based on a set of repeating properties. • The periodic table allows you to easily compare the properties of one element to another. • Each horizontal row is called a period. • Each vertical column is called a group, or family. – Elements in a group typica ...
EMR and the Bohr Model of the Atom
... • Related to the shape of the atomic orbitals • This quantum number depends on the value of n. The values of l begin at 0 and increase to (n - 1). • Because we use numbers to describe the first quantum number, we usually use letters for l (s for l =0, p for l = 1, d for l =2 and f for l = 3). • Usua ...
... • Related to the shape of the atomic orbitals • This quantum number depends on the value of n. The values of l begin at 0 and increase to (n - 1). • Because we use numbers to describe the first quantum number, we usually use letters for l (s for l =0, p for l = 1, d for l =2 and f for l = 3). • Usua ...
STRUCTURE OF AN ATOM
... Atom consist of two parts: (a)Nucleus: Almost the whole mass of the atom and positive charge is concentrated in this small region (b)Extra nuclear part:this is the space around the nucleus in which electrons are revolving at high speeds in fixed path © Electrons and nucleus are held together by elec ...
... Atom consist of two parts: (a)Nucleus: Almost the whole mass of the atom and positive charge is concentrated in this small region (b)Extra nuclear part:this is the space around the nucleus in which electrons are revolving at high speeds in fixed path © Electrons and nucleus are held together by elec ...
Chapter 4
... – Single e- circled around nucleus in allowed paths or orbits – e- has fixed E when in this orbit (lowest E closest to nucleus) – Lot of empty space between nucleus and e- in which e- cannot be in – E increases as e- moves to farther orbits – http://chemmovies.unl.edu/ChemAnime/BOHRQD/B OHRQD.html ...
... – Single e- circled around nucleus in allowed paths or orbits – e- has fixed E when in this orbit (lowest E closest to nucleus) – Lot of empty space between nucleus and e- in which e- cannot be in – E increases as e- moves to farther orbits – http://chemmovies.unl.edu/ChemAnime/BOHRQD/B OHRQD.html ...
Lecture 2 - Tufts University
... • For an atom, use Schrödinger’s equation • Find permissible energy levels for electrons around nucleus. • For each energy level, the wave function defines an orbital, a region where the probability of finding an electron is high • The orbital properties of greatest interest are size, shape (describ ...
... • For an atom, use Schrödinger’s equation • Find permissible energy levels for electrons around nucleus. • For each energy level, the wave function defines an orbital, a region where the probability of finding an electron is high • The orbital properties of greatest interest are size, shape (describ ...
Corso di Fisica Moderna
... 2) Instead of the infinity of orbits which would be possible in classical mechanics, it is only possible for an electron to move in an orbit for which its orbital angular momentum L is and integra ...
... 2) Instead of the infinity of orbits which would be possible in classical mechanics, it is only possible for an electron to move in an orbit for which its orbital angular momentum L is and integra ...
Lecture 18: Intro. to Quantum Mechanics
... • Another limitation of the Bohr model was that it assumed we could know both the position and momentum of an electron exactly. • Werner Heisenberg development of quantum mechanics leads him to the observation that there is a fundamental limit to how well one can know both the position and momentum ...
... • Another limitation of the Bohr model was that it assumed we could know both the position and momentum of an electron exactly. • Werner Heisenberg development of quantum mechanics leads him to the observation that there is a fundamental limit to how well one can know both the position and momentum ...
5.4 Quantum Devices Energy Levels in a Single Quantum Well
... Since single atoms may also be described as SQWs (for one electron you just have the hydrogen atom type with a Coulomb potential), we must expect that the wave function of the electrons start to overlap as soon as the single SQWs in the MQW structure are close enough. The situation is completely ana ...
... Since single atoms may also be described as SQWs (for one electron you just have the hydrogen atom type with a Coulomb potential), we must expect that the wave function of the electrons start to overlap as soon as the single SQWs in the MQW structure are close enough. The situation is completely ana ...