File - Chemistry 11 Enriched
... understand the location of electrons, we must now look at the atom in three dimensions rather than the planetary early model of the atom. The orbitals are not two dimensional tracks like railroads circling an atom, but are rather areas of three dimensional space where we expect to find the electron. ...
... understand the location of electrons, we must now look at the atom in three dimensions rather than the planetary early model of the atom. The orbitals are not two dimensional tracks like railroads circling an atom, but are rather areas of three dimensional space where we expect to find the electron. ...
Lecture 4
... There are letters associated with values of orbital angular momentum. The first few are: For example, state with n=1 l=0 is referred to as 1s, n=2 l=0 is referred to as 2s, n=2 l=1 is referred to as 2p, and so on. While the energies are the same for the four n=2 states, the wave functions are not: ...
... There are letters associated with values of orbital angular momentum. The first few are: For example, state with n=1 l=0 is referred to as 1s, n=2 l=0 is referred to as 2s, n=2 l=1 is referred to as 2p, and so on. While the energies are the same for the four n=2 states, the wave functions are not: ...
Atomic Theory
... Within a given atom, the lower the value of n, the more stable (or lower in energy) the orbital There are n types of orbitals in the nth energy level There are 2l + 1 orbitals of each type There are n - l - 1 nodes in the radial distribution functions of all orbitals (spherical nodes) Ther ...
... Within a given atom, the lower the value of n, the more stable (or lower in energy) the orbital There are n types of orbitals in the nth energy level There are 2l + 1 orbitals of each type There are n - l - 1 nodes in the radial distribution functions of all orbitals (spherical nodes) Ther ...
De Broglie waves
... – r: the length of radius vector from origin O to point P; – θ: zenith angle, angle between radius vector and +z axis, – φ: azimuth angle, angle between the projection of the radius vector in xy plane and the +x axis, ...
... – r: the length of radius vector from origin O to point P; – θ: zenith angle, angle between radius vector and +z axis, – φ: azimuth angle, angle between the projection of the radius vector in xy plane and the +x axis, ...
Snímek 1 - Fordham University Computer and Information Sciences
... Current analytical formulas for the propagation of an electron in the Quantum regime through a potential barrier assumes many electrons and the position of any one individual electron is unimportant, but rather their behavior as a group is studied. As a result, description of current is always in te ...
... Current analytical formulas for the propagation of an electron in the Quantum regime through a potential barrier assumes many electrons and the position of any one individual electron is unimportant, but rather their behavior as a group is studied. As a result, description of current is always in te ...
Chemistry 1 Practice Final Exam - Tutor
... n = 2, l = 1, ml = 1 : n = 4, l = 2, ml = -2 : n = 3, l = 1, ml = 2 : n = 2, l = 2, ml = -1 ...
... n = 2, l = 1, ml = 1 : n = 4, l = 2, ml = -2 : n = 3, l = 1, ml = 2 : n = 2, l = 2, ml = -1 ...
Lecture 29B - UCSD Department of Physics
... The electron cloud model is quite different from the Bohr model. The electron cloud structure does not change with time and remains the same on average. The atom does not radiate when it is in one particular quantum state. This removes the problem of the Rutherford atom. Radiative transition causes ...
... The electron cloud model is quite different from the Bohr model. The electron cloud structure does not change with time and remains the same on average. The atom does not radiate when it is in one particular quantum state. This removes the problem of the Rutherford atom. Radiative transition causes ...
Spectroscopy
... determine R experimentally by observing the emission spectrum of Hydrogen. The four bright spectral lines in the visible portion of the EM-spectrum arise from transitions from the n = 3, 4, 5, & 6 energy levels to the n = 2 level. We will measure the frequencies of those four lines, and from that da ...
... determine R experimentally by observing the emission spectrum of Hydrogen. The four bright spectral lines in the visible portion of the EM-spectrum arise from transitions from the n = 3, 4, 5, & 6 energy levels to the n = 2 level. We will measure the frequencies of those four lines, and from that da ...
Pdf
... provide a static negative charge cloud that is spatially distributed according to the extension of the orbitals comprised in (⫽0). The exchange operators reduce the magnitude of the corresponding repulsion somewhat, but only to a partial extent. As increases from 0 to 1 in H(), Eq. 共2.2兲, this ...
... provide a static negative charge cloud that is spatially distributed according to the extension of the orbitals comprised in (⫽0). The exchange operators reduce the magnitude of the corresponding repulsion somewhat, but only to a partial extent. As increases from 0 to 1 in H(), Eq. 共2.2兲, this ...
Schrodinger models of the atom
... Quantum mechanics places the electrons in orbitals, not fixed orbits. Orbitals are regions of space. The electrons are like a cloud of negative charge within that orbital. The electron shells proposed by Bohr are still used, but the electrons in each shell are not all equal in energy. The shell has ...
... Quantum mechanics places the electrons in orbitals, not fixed orbits. Orbitals are regions of space. The electrons are like a cloud of negative charge within that orbital. The electron shells proposed by Bohr are still used, but the electrons in each shell are not all equal in energy. The shell has ...
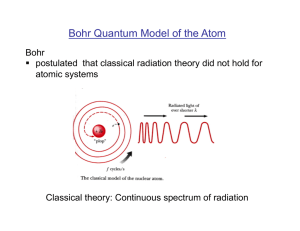
Bohr Quantum Model of the Atom
... § postulated that the electron orbital momentum is quantized Justification of Bohr’s postulates: comparison with experimental observations! ...
... § postulated that the electron orbital momentum is quantized Justification of Bohr’s postulates: comparison with experimental observations! ...
Chapter 6 and 7 Reading Guide Electronic Structure of Atoms and
... Be sure to read “A Closer Look” on page 260. Follow the diagram for a clear understanding of effective nuclear charge. This concept is critical to understanding periodic trends. Section 7.3 Define the following terms: non-bonding atomic radius (van der Waal’s radius): ...
... Be sure to read “A Closer Look” on page 260. Follow the diagram for a clear understanding of effective nuclear charge. This concept is critical to understanding periodic trends. Section 7.3 Define the following terms: non-bonding atomic radius (van der Waal’s radius): ...
Chapter 5: The Quantum Mechanical Model of the Atom I. The
... D. The Bohr Model of the Atom: 1. Bohr s major idea was that the energy states of the atom were _________, and that the amount of energy in the atom was related to the electron s position in the atom. 2. The electrons travel in orbits that are at a fixed distance from the nucleus. ...
... D. The Bohr Model of the Atom: 1. Bohr s major idea was that the energy states of the atom were _________, and that the amount of energy in the atom was related to the electron s position in the atom. 2. The electrons travel in orbits that are at a fixed distance from the nucleus. ...