Chemistry Semester 2 Final Exam Chemistry Semester 2 Final Exam
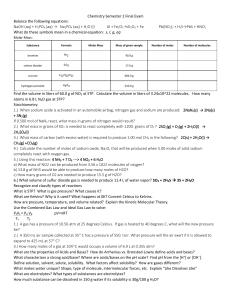
... 1.) A gas has a pressure of 10.56 atm at 25 degrees Celcius. If gas is heated to 40 degrees C, what will the new pressure be? 10.0 atm 2.) A 350 mL air sample collected at 35 C has a pressure of 550. torr. What pressure will the air exert if it is allowed to expand to 425 mL at 57 C? 485 torr 3.) ...
... 1.) A gas has a pressure of 10.56 atm at 25 degrees Celcius. If gas is heated to 40 degrees C, what will the new pressure be? 10.0 atm 2.) A 350 mL air sample collected at 35 C has a pressure of 550. torr. What pressure will the air exert if it is allowed to expand to 425 mL at 57 C? 485 torr 3.) ...
Discussion 9, Mahaffy et al., Chapter 15
... b. With regards to CaF2, circle the correct choice Precipitation will occur Precipitation will not occur More information needed 5. The number of moles of a solid that dissolves in 1 liter of water is called the molar solubility. For Ag2SO4, the molar solubility is 1.4∙10-2 M. What is the Ksp ...
... b. With regards to CaF2, circle the correct choice Precipitation will occur Precipitation will not occur More information needed 5. The number of moles of a solid that dissolves in 1 liter of water is called the molar solubility. For Ag2SO4, the molar solubility is 1.4∙10-2 M. What is the Ksp ...
Chapter 23 (Section 3) Pregnancy, Birth, and
... a. each ELEMENT has its own CHARACTERISTIC, chemical, and PHYSICAL properties *b. elements can NOT be BROKEN down into other substances by any CHEMICAL means c. some MATTER exists in elemental form [(e.g.) gold [Au] = not chemically REACTIVE)] *d. ELEMENTS individually or combined form everything in ...
... a. each ELEMENT has its own CHARACTERISTIC, chemical, and PHYSICAL properties *b. elements can NOT be BROKEN down into other substances by any CHEMICAL means c. some MATTER exists in elemental form [(e.g.) gold [Au] = not chemically REACTIVE)] *d. ELEMENTS individually or combined form everything in ...
Ch 12 Solutions
... to form the ionic crystal. It is the result of ionic bonds. - Ionic solids dissolve when hydration energy released is greater than lattice energy absorbed. - In general, compounds with singly-charged ions, like Na+1 and K+1, are very soluble in water because they have relatively small lattice energi ...
... to form the ionic crystal. It is the result of ionic bonds. - Ionic solids dissolve when hydration energy released is greater than lattice energy absorbed. - In general, compounds with singly-charged ions, like Na+1 and K+1, are very soluble in water because they have relatively small lattice energi ...
Density, Viscosity, Solubility, and Diffusivity of N2O in Aqueous
... removal of acid gas components such as CO2 and H2S from a variety of industrial gas streams.1 In particular, alkanolamines and blends of alkanolamines are widely used amines in the gas-treating industry. Alkanolamines undergo degradation in oxygen-rich atmosphere, usually encountered in the treatmen ...
... removal of acid gas components such as CO2 and H2S from a variety of industrial gas streams.1 In particular, alkanolamines and blends of alkanolamines are widely used amines in the gas-treating industry. Alkanolamines undergo degradation in oxygen-rich atmosphere, usually encountered in the treatmen ...
Chapter 6
... completely dissociate and so will strong Arrhenius bases (those that produce in solution). Weak Electrolytes are: ...
... completely dissociate and so will strong Arrhenius bases (those that produce in solution). Weak Electrolytes are: ...
Chapter 7 lecture notes: Solutions
... This particular gas producing reaction is important in medicine because sodium bicarbonate is used as an over-the-counter therapeutic agent to treat acid indigestion (heartburn). Sodium bicarbonate is the primary active ingredient in many antacids, such as alka-seltzer. Sodium bicarbonate “neutraliz ...
... This particular gas producing reaction is important in medicine because sodium bicarbonate is used as an over-the-counter therapeutic agent to treat acid indigestion (heartburn). Sodium bicarbonate is the primary active ingredient in many antacids, such as alka-seltzer. Sodium bicarbonate “neutraliz ...
File
... • C12H22O11 (s) C12H22O11 (aq) • NO dissociation because NO ions • Sucrose dissolves in water because sugar is polar (-OH group), but dissociation does not occur. Sucrose molecules are simply separated from each other. No ions are formed ...
... • C12H22O11 (s) C12H22O11 (aq) • NO dissociation because NO ions • Sucrose dissolves in water because sugar is polar (-OH group), but dissociation does not occur. Sucrose molecules are simply separated from each other. No ions are formed ...
Answers PRACTICE EXAM II Spring 2008 Part I. Multiple Choice (3
... 8. Which one of the following best describes what occurs in a reaction system when it reaches a state of dynamic equilibrium? 4. the rates for both forward and reverse reaction processes are the same 9. Which of the following solutions has the lowest freezing point? 3. 1.0 m Na2SO4 in water 10. Ide ...
... 8. Which one of the following best describes what occurs in a reaction system when it reaches a state of dynamic equilibrium? 4. the rates for both forward and reverse reaction processes are the same 9. Which of the following solutions has the lowest freezing point? 3. 1.0 m Na2SO4 in water 10. Ide ...
Contents and Concepts Learning Objectives
... Solubility and the Common-Ion Effect • In this section we will look at calculating solubilities in the presence of other ions. – The importance of the Ksp becomes apparent when you consider the solubility of one salt in the solution of another having the same cation. ...
... Solubility and the Common-Ion Effect • In this section we will look at calculating solubilities in the presence of other ions. – The importance of the Ksp becomes apparent when you consider the solubility of one salt in the solution of another having the same cation. ...
Solubility and Complex-ion Equilibria
... separating two or more ions from a solution by adding a reactant that precipitates first one ion, then another, and so forth. – After most of the Ba2+ ion has precipitated, strontium chromate begins to precipitate. – It is therefore possible to separate Ba2+ from Sr2+ by ...
... separating two or more ions from a solution by adding a reactant that precipitates first one ion, then another, and so forth. – After most of the Ba2+ ion has precipitated, strontium chromate begins to precipitate. – It is therefore possible to separate Ba2+ from Sr2+ by ...
Chemical Equilibrium
... • The equilibrium constant (K) is the ratio of the mathematical product of the concentrations of substances formed at equilibrium to the mathematical product of the concentrations of reacting substances. Each concentration is raised to a power equal to the coefficient of that substance in the chemic ...
... • The equilibrium constant (K) is the ratio of the mathematical product of the concentrations of substances formed at equilibrium to the mathematical product of the concentrations of reacting substances. Each concentration is raised to a power equal to the coefficient of that substance in the chemic ...
Zumdahl`s Chap. 4 - The University of Texas at Dallas
... Weak Electrolytes are mostly molecular. ...
... Weak Electrolytes are mostly molecular. ...
Chap18 - Bakersfield College
... separating two or more ions from a solution by adding a reactant that precipitates first one ion, then another, and so forth. – After most of the Ba2+ ion has precipitated, strontium chromate begins to precipitate. – It is therefore possible to separate Ba2+ from Sr2+ by ...
... separating two or more ions from a solution by adding a reactant that precipitates first one ion, then another, and so forth. – After most of the Ba2+ ion has precipitated, strontium chromate begins to precipitate. – It is therefore possible to separate Ba2+ from Sr2+ by ...
Contents and Concepts Learning Objectives
... • Fractional precipitation is the technique of separating two or more ions from a solution by adding a reactant that precipitates first one ion, then another, and so forth. – After most of the Ba2+ ion has precipitated, strontium chromate begins to precipitate. – It is therefore possible to separate ...
... • Fractional precipitation is the technique of separating two or more ions from a solution by adding a reactant that precipitates first one ion, then another, and so forth. – After most of the Ba2+ ion has precipitated, strontium chromate begins to precipitate. – It is therefore possible to separate ...
Contents and Concepts Learning Objectives
... separating two or more ions from a solution by adding a reactant that precipitates first one ion, then another, and so forth. – After most of the Ba2+ ion has precipitated, strontium chromate begins to precipitate. – It is therefore possible to separate Ba2+ from Sr2+ by ...
... separating two or more ions from a solution by adding a reactant that precipitates first one ion, then another, and so forth. – After most of the Ba2+ ion has precipitated, strontium chromate begins to precipitate. – It is therefore possible to separate Ba2+ from Sr2+ by ...
1412_lecture_ch16 Fall_2014
... Complex-Ion Formation The formation constant, Kf, is the equilibrium constant for the formation of a complex ion from the aqueous metal ion and the ligands. The large value means that the complex ion is quite stable. When a large amount of NH3 is added to a solution of Ag+, you expect most of the A ...
... Complex-Ion Formation The formation constant, Kf, is the equilibrium constant for the formation of a complex ion from the aqueous metal ion and the ligands. The large value means that the complex ion is quite stable. When a large amount of NH3 is added to a solution of Ag+, you expect most of the A ...
Chapter 4
... All compounds containing alkali metal cations and the ammonium ion are soluble. All compounds containing NO3-, ClO4-, ClO3-, and C2H3O2- anions are soluble. All chlorides, bromides, and iodides are soluble except those containing Ag+, Pb2+, and Hg2+. All sulfates are soluble except those containing ...
... All compounds containing alkali metal cations and the ammonium ion are soluble. All compounds containing NO3-, ClO4-, ClO3-, and C2H3O2- anions are soluble. All chlorides, bromides, and iodides are soluble except those containing Ag+, Pb2+, and Hg2+. All sulfates are soluble except those containing ...
Solubility and Complex-ion Equilibria
... separating two or more ions from a solution by adding a reactant that precipitates first one ion, then another, and so forth. – After most of the Ba2+ ion has precipitated, strontium chromate begins to precipitate. – It is therefore possible to separate Ba2+ from Sr2+ by ...
... separating two or more ions from a solution by adding a reactant that precipitates first one ion, then another, and so forth. – After most of the Ba2+ ion has precipitated, strontium chromate begins to precipitate. – It is therefore possible to separate Ba2+ from Sr2+ by ...
Solubility and Complex-ion Equilibria
... separating two or more ions from a solution by adding a reactant that precipitates first one ion, then another, and so forth. – After most of the Ba2+ ion has precipitated, strontium chromate begins to precipitate. – It is therefore possible to separate Ba2+ from Sr2+ by ...
... separating two or more ions from a solution by adding a reactant that precipitates first one ion, then another, and so forth. – After most of the Ba2+ ion has precipitated, strontium chromate begins to precipitate. – It is therefore possible to separate Ba2+ from Sr2+ by ...
Candidates should check the question paper to
... b) In another experiment, a gas jar containing moist sulphur (IV) oxide is inverted over another gas jar containing hydrogen sulphide gas. State and explain the observation that is ...
... b) In another experiment, a gas jar containing moist sulphur (IV) oxide is inverted over another gas jar containing hydrogen sulphide gas. State and explain the observation that is ...
Contents and Concepts Learning Objectives
... • The solubility of an insoluble salt can be manipulated by adding a species that reacts with either the cation or the anion. • Effect of pH on Solubility • When a salt contains the conjugate base of a weak acid, the pH will affect the solubility of the salt. ...
... • The solubility of an insoluble salt can be manipulated by adding a species that reacts with either the cation or the anion. • Effect of pH on Solubility • When a salt contains the conjugate base of a weak acid, the pH will affect the solubility of the salt. ...