ic199p5a
... (b) Explain why KNO3(s) is highly soluble in water despite this observation regarding the temperature change on solution. If it is readily soluble, the G for solution is likely to be negative, which means the increase in entropy on dissolution (the TS term) is sufficient to overcome the positive ...
... (b) Explain why KNO3(s) is highly soluble in water despite this observation regarding the temperature change on solution. If it is readily soluble, the G for solution is likely to be negative, which means the increase in entropy on dissolution (the TS term) is sufficient to overcome the positive ...
AP Chemistry Summer Assignment 2016
... 1) Name these ionic compounds: Ca(NO3)2, SnO, CuCr2O7, Al(CN)3, HCl (aq), (NH4)2SO4, CrCO3, NiF2, NaH, BaO2, Fe(OH)3, Ag2CrO4, Cr2(HPO4)3, KClO4, Ba(SCN)2 2) Write the correct chemical formulas of these compounds: cadmium bicarbonate, plumbous chloride, aluminum oxide, copper (I) cyanide, mercury (I ...
... 1) Name these ionic compounds: Ca(NO3)2, SnO, CuCr2O7, Al(CN)3, HCl (aq), (NH4)2SO4, CrCO3, NiF2, NaH, BaO2, Fe(OH)3, Ag2CrO4, Cr2(HPO4)3, KClO4, Ba(SCN)2 2) Write the correct chemical formulas of these compounds: cadmium bicarbonate, plumbous chloride, aluminum oxide, copper (I) cyanide, mercury (I ...
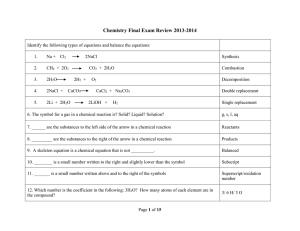
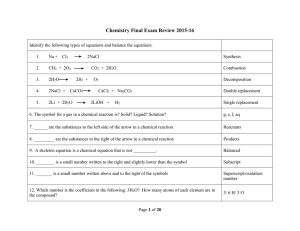
Type Of Chemical Reaction
... A strong base is typically a pH of what number? _____14_____ The ion that accounts for Acidity is: _____H+_or H3O+____ The ion that accounts for Basic is: ____OH-____ ...
... A strong base is typically a pH of what number? _____14_____ The ion that accounts for Acidity is: _____H+_or H3O+____ The ion that accounts for Basic is: ____OH-____ ...
Ch 7: Reactions
... • For example, consider the following compounds; NaCl, BaSO4, NaC2H3O2, and CaS. Determine the solubility in water for these ionic substances. • NaCl (all chlorides are soluble except...) SOLUBLE = aqueous • BaSO4 (all sulfates are soluble except...) INSOLUBLE = solid • NaC2H3O2 (all sodium compound ...
... • For example, consider the following compounds; NaCl, BaSO4, NaC2H3O2, and CaS. Determine the solubility in water for these ionic substances. • NaCl (all chlorides are soluble except...) SOLUBLE = aqueous • BaSO4 (all sulfates are soluble except...) INSOLUBLE = solid • NaC2H3O2 (all sodium compound ...
Type Of Chemical Reaction
... A strong base is typically a pH of what number? _____14_____ The ion that accounts for Acidity is: _____H+_or H3O+____ The ion that accounts for Basic is: ____OH-____ ...
... A strong base is typically a pH of what number? _____14_____ The ion that accounts for Acidity is: _____H+_or H3O+____ The ion that accounts for Basic is: ____OH-____ ...
CHEM1100 Practice Exam 2 You have 120 minutes to complete this
... 9. Calculate the percentage mass composition of each element in ammonia (NH3). ...
... 9. Calculate the percentage mass composition of each element in ammonia (NH3). ...
_______1. solution a. capable of being dissolved _______2. solute
... a. capable of being dissolved ...
... a. capable of being dissolved ...
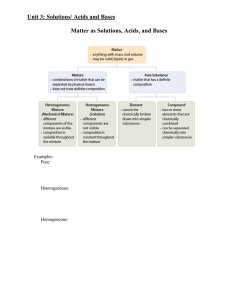
Chemical Reaction and Matter Review
... A chemical formula is a combination of elemental symbols and subscript numbers that is used to show the composition of a compound. Depending of the type of compound that the formula represents, the information that it provides will vary slightly. Before we go about learning how to write chemical for ...
... A chemical formula is a combination of elemental symbols and subscript numbers that is used to show the composition of a compound. Depending of the type of compound that the formula represents, the information that it provides will vary slightly. Before we go about learning how to write chemical for ...
الشريحة 1
... themselves on the surface of the NaCl crystals. The +ve end of H2O dipole is oriented toward the Clions, and the –ve end of the H2O dipole is oriented toward the Na+ ions. The ion-dipole attractions between the ions and H2O molecules are strong enough to pull the ions from their positions in the cry ...
... themselves on the surface of the NaCl crystals. The +ve end of H2O dipole is oriented toward the Clions, and the –ve end of the H2O dipole is oriented toward the Na+ ions. The ion-dipole attractions between the ions and H2O molecules are strong enough to pull the ions from their positions in the cry ...
Dissociation
... — However, general solubility guidelines do exist, which offer information in general about what substances will dissolve in water — Learn to use your reference tables — it’s fun and if you take advantage of this special limited time offer, it’s absolutely free — The guidelines are useful in helping ...
... — However, general solubility guidelines do exist, which offer information in general about what substances will dissolve in water — Learn to use your reference tables — it’s fun and if you take advantage of this special limited time offer, it’s absolutely free — The guidelines are useful in helping ...
Outline for Unit 1 Solutions, Acid/Base, and Gases
... 3. Temperature – at higher temp kinetic energy of the solvent is higher so more collisions of solvent molecules with solute 4. Particle size – smaller particles dissolve faster since there is more surface area available to solvent 5. Pressure (partial pressure) – only affects gas in liquids – solubi ...
... 3. Temperature – at higher temp kinetic energy of the solvent is higher so more collisions of solvent molecules with solute 4. Particle size – smaller particles dissolve faster since there is more surface area available to solvent 5. Pressure (partial pressure) – only affects gas in liquids – solubi ...
Matter - tompkinsmath
... a) Elements – substances composed of only one kind of atom which cannot be broken down using heat or electricity. Ex. Na, Br, O2, S8 b) Compounds – substances composed of 2 or more kinds of atoms and can be decomposed using heat or electricity. Ex. H2O, NaCl, C12H22O11 Mixtures – mixtures of pure su ...
... a) Elements – substances composed of only one kind of atom which cannot be broken down using heat or electricity. Ex. Na, Br, O2, S8 b) Compounds – substances composed of 2 or more kinds of atoms and can be decomposed using heat or electricity. Ex. H2O, NaCl, C12H22O11 Mixtures – mixtures of pure su ...
solution is a solution that contains the maximum amount of solute
... Part A: Fill-in-the-blanks. Choose the word that best completes each statement. [36 points/2 points each] ...
... Part A: Fill-in-the-blanks. Choose the word that best completes each statement. [36 points/2 points each] ...
2015 Academic Challenge CHEMISTRY TEST – STATE
... a solution that has too much solute for a given temperature. a mixture in which there is more solute than solvent. a solution in which the solvent has dissolved the maximum amount possible of a given solute at a given temperature. E. none of the above describes a saturated solution. ...
... a solution that has too much solute for a given temperature. a mixture in which there is more solute than solvent. a solution in which the solvent has dissolved the maximum amount possible of a given solute at a given temperature. E. none of the above describes a saturated solution. ...
Chapter 7: Solutions
... the difference between solute(s) and solvent. Predict the effect of temperature and pressure on the solubility of gases in water and the effect of temperature on the solubility of solids in water. Be able to use the Solubility Rules Table to determine if an ionic compound will significantly dissolve ...
... the difference between solute(s) and solvent. Predict the effect of temperature and pressure on the solubility of gases in water and the effect of temperature on the solubility of solids in water. Be able to use the Solubility Rules Table to determine if an ionic compound will significantly dissolve ...
CHEM 301: AQUEOUS ENVIRONMENTAL CHEMISTRY
... Ions that are abundant in the geosphere and highly stable as dissolved species tend to accumulate in natural waters. Na+, K+, Ca2+, Mg2+, Cl-, SO42-, NO3-, HCO3All aqueous solutions are electrically neutral and the total positive charge on all cations is balanced by the total negative charge on all ...
... Ions that are abundant in the geosphere and highly stable as dissolved species tend to accumulate in natural waters. Na+, K+, Ca2+, Mg2+, Cl-, SO42-, NO3-, HCO3All aqueous solutions are electrically neutral and the total positive charge on all cations is balanced by the total negative charge on all ...
Chapter 4: Solution Chemistry and the Hydrosphere
... • A concentrated solution has a large quantity of solute present for a given amount of solution. • A dilute solution has a small quantity of solute present for a given amount of solution. amount of solute amount of solvent The more solute in a given amount of solution the more concentrated the sol ...
... • A concentrated solution has a large quantity of solute present for a given amount of solution. • A dilute solution has a small quantity of solute present for a given amount of solution. amount of solute amount of solvent The more solute in a given amount of solution the more concentrated the sol ...
Reactions in Aqueous Solution (Brown 13th-Fossum
... Generally, when solutions of an acid and a base are combined, the products are a salt and water. CH3COOH (aq) + NaOH (aq) CH3COONa (aq) + H2O (l) When a strong acid reacts with a strong base, the net ionic equation is… HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) H+ (aq) + OH- (aq) H2O (l) Polyp ...
... Generally, when solutions of an acid and a base are combined, the products are a salt and water. CH3COOH (aq) + NaOH (aq) CH3COONa (aq) + H2O (l) When a strong acid reacts with a strong base, the net ionic equation is… HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) H+ (aq) + OH- (aq) H2O (l) Polyp ...
Chemistry Lab 2010
... Factors that influence solubility • Polarity of Solute and Solvent – “Like Dissolves Like” Polar solutes dissolve in polar solvents Nonpolar solutes dissolve in nonpolar solvents Nonpolar solutes do not dissolve well in polar solvents • Temperature Solubility of most solids in water increases with ...
... Factors that influence solubility • Polarity of Solute and Solvent – “Like Dissolves Like” Polar solutes dissolve in polar solvents Nonpolar solutes dissolve in nonpolar solvents Nonpolar solutes do not dissolve well in polar solvents • Temperature Solubility of most solids in water increases with ...
Cheat Sheet for Chemical Equilibrium
... • Given: Initial Concentration of Reactants only‐ Products will be zero. Determine the change by subtracting “x” from reactants and adding “x” to products. • Given: Initial Concentrations of Products only‐ Reactants will be zero. Determine the change by subtracting “x” from the products and addin ...
... • Given: Initial Concentration of Reactants only‐ Products will be zero. Determine the change by subtracting “x” from reactants and adding “x” to products. • Given: Initial Concentrations of Products only‐ Reactants will be zero. Determine the change by subtracting “x” from the products and addin ...
Chapter 4 Reactions in Aqueous Solution 4.1 Aqueous Solutions
... • Chemical Equilibrium - when reactants form products as fast as products form reactants, no further net change in concentrations ...
... • Chemical Equilibrium - when reactants form products as fast as products form reactants, no further net change in concentrations ...
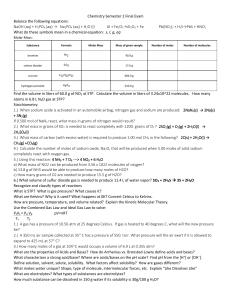
Chemistry Semester 2 Final Exam Chemistry Semester 2 Final Exam
... 1.) A gas has a pressure of 10.56 atm at 25 degrees Celcius. If gas is heated to 40 degrees C, what will the new pressure be? 10.0 atm 2.) A 350 mL air sample collected at 35 C has a pressure of 550. torr. What pressure will the air exert if it is allowed to expand to 425 mL at 57 C? 485 torr 3.) ...
... 1.) A gas has a pressure of 10.56 atm at 25 degrees Celcius. If gas is heated to 40 degrees C, what will the new pressure be? 10.0 atm 2.) A 350 mL air sample collected at 35 C has a pressure of 550. torr. What pressure will the air exert if it is allowed to expand to 425 mL at 57 C? 485 torr 3.) ...