3.2 “Conserving” Energy
... • The specific heat is a property of a substance that tells us how much heat is needed to raise the temperature of one kilogram of a material by one degree Celsius. Knowing the specific heat of a material tells you how quickly the temperature will change as it gains or loses energy. ...
... • The specific heat is a property of a substance that tells us how much heat is needed to raise the temperature of one kilogram of a material by one degree Celsius. Knowing the specific heat of a material tells you how quickly the temperature will change as it gains or loses energy. ...
Chapter 2 - UCSB Chemical Engineering
... is approximately equal to the enthalpy, Uint H, and H depends only on temperature. Consequently, in the subsequent development, we assume that Uint = H and Uˆ int Hˆ where the caret (^) means per unit mass. As shown in Appendix B, a differential change in temperature, dT, produces a correspondin ...
... is approximately equal to the enthalpy, Uint H, and H depends only on temperature. Consequently, in the subsequent development, we assume that Uint = H and Uˆ int Hˆ where the caret (^) means per unit mass. As shown in Appendix B, a differential change in temperature, dT, produces a correspondin ...
energy
... Organized form of energy is more valuable than the disorganized form of energy. Organized energy can be converted to disorganized energy completely. Only fraction of disorganized energy can be converted into organized energy by specially built devices called heat engines. Thermodynamics: the convers ...
... Organized form of energy is more valuable than the disorganized form of energy. Organized energy can be converted to disorganized energy completely. Only fraction of disorganized energy can be converted into organized energy by specially built devices called heat engines. Thermodynamics: the convers ...
The First Law of Thermodynamics Chapter 19
... – Internal energy, concept of state variables – Difference between Work and Heat • II. Examine various types of thermodynamic processes: – Constant volume – Constant pressure – Constant temperature – Zero heat transfer - adiabatic process Copyright © 2008 Pearson Education Inc., publishing as ...
... – Internal energy, concept of state variables – Difference between Work and Heat • II. Examine various types of thermodynamic processes: – Constant volume – Constant pressure – Constant temperature – Zero heat transfer - adiabatic process Copyright © 2008 Pearson Education Inc., publishing as ...
Original Stirling Engine demonstration proposal
... engine that operates between the hot (TH) and cold (TC) reservoirs. For each cyclic process, heat QH is added from the hot reservoir (TH) to the engine and the engine does work W by using that heat. Not all of the heat QH is converted to work and the left over energy QC = QH – W leaves the engine an ...
... engine that operates between the hot (TH) and cold (TC) reservoirs. For each cyclic process, heat QH is added from the hot reservoir (TH) to the engine and the engine does work W by using that heat. Not all of the heat QH is converted to work and the left over energy QC = QH – W leaves the engine an ...
Fundamentals of chemical thermodynamics and bioenergetics
... atmosphere, when the mixture is sprayed into the atmosphere it expands so rapidly that, as a good approximation, no heat exchange occurs between the system (air and water) and its surroundings; that is, Q = 0. (In thermodynamics, such a process is called an adiabatic process.) Thus, we write ΔU = Q ...
... atmosphere, when the mixture is sprayed into the atmosphere it expands so rapidly that, as a good approximation, no heat exchange occurs between the system (air and water) and its surroundings; that is, Q = 0. (In thermodynamics, such a process is called an adiabatic process.) Thus, we write ΔU = Q ...
JIF 314 Thermodynamics - comsics
... system of a whole since different parts of the system are thermodynamically inequivalent. • A single value of e.g. temperature T = 300 K is insufficient to account for the temperature of the system as a whole since in different parts of a system in non-TE the temperature are different. ...
... system of a whole since different parts of the system are thermodynamically inequivalent. • A single value of e.g. temperature T = 300 K is insufficient to account for the temperature of the system as a whole since in different parts of a system in non-TE the temperature are different. ...
Name Section
... available to do additional work. Perpetual motion does not exist. Since some energy is wasted in every process, that energy must be replaced to keep a machine working. Perpetual motion would violate the second law of thermodynamics, which states that all physical processes are irreversible. ...
... available to do additional work. Perpetual motion does not exist. Since some energy is wasted in every process, that energy must be replaced to keep a machine working. Perpetual motion would violate the second law of thermodynamics, which states that all physical processes are irreversible. ...
Thermodynamic Symbols and Constants
... Thermodynamic Symbols and Constants Definition of Thermodynamic Symbols E is the intrisic energy; (J/mol) H is the enthalpy (= E + PV); (J/mol) G is the Gibbs energy (= H – TS); (J/mol) Cp is the heat capacity at constant temperature; (J/mol K) The superscript o denotes the value given is for the st ...
... Thermodynamic Symbols and Constants Definition of Thermodynamic Symbols E is the intrisic energy; (J/mol) H is the enthalpy (= E + PV); (J/mol) G is the Gibbs energy (= H – TS); (J/mol) Cp is the heat capacity at constant temperature; (J/mol K) The superscript o denotes the value given is for the st ...
Chapter 12: Thermodynamic Property Relations
... volumetric expansion) is a measure of the change in volume with temperature at constant pressure. ...
... volumetric expansion) is a measure of the change in volume with temperature at constant pressure. ...
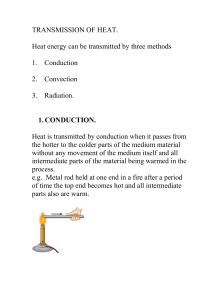
Lecture 5: Heat transmission
... P = Heat loss rating = Rate of energy flow through the element = Power measured in Watts. A = Area of the material measured in m2. T = temperature difference measured in oC or Kelvin ...
... P = Heat loss rating = Rate of energy flow through the element = Power measured in Watts. A = Area of the material measured in m2. T = temperature difference measured in oC or Kelvin ...
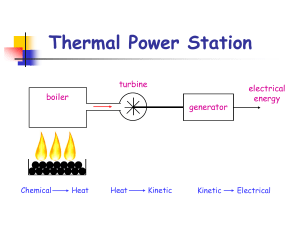
Thermal Power Station
... In a pumped hydroelectric power station water can be pumped back-up from the low level reservoir to the higher level. This usually happens throughout the night when the demand for electricity is lower. In the morning when we wake up and there is a demand for electricity, the water is allowed to flow ...
... In a pumped hydroelectric power station water can be pumped back-up from the low level reservoir to the higher level. This usually happens throughout the night when the demand for electricity is lower. In the morning when we wake up and there is a demand for electricity, the water is allowed to flow ...
Ppt19(PS8)_Thermo_Hess
... system (and into the surroundings) & qsurr > 0 • qsys > 0 means heat flowed INTO the system (and out of the surroundings) & qsurr < 0 • E.g.: If 10 J flows from sys to surr: qsys = -10 J and qsurr = +10 J ...
... system (and into the surroundings) & qsurr > 0 • qsys > 0 means heat flowed INTO the system (and out of the surroundings) & qsurr < 0 • E.g.: If 10 J flows from sys to surr: qsys = -10 J and qsurr = +10 J ...
SMS-204: Integrative marine sciences.
... Friction is the process through which mechanical energy is converted to heat. The breaks in our car heat when we use them and so do our hands when we rub them against each other. Friction can often be an undesirable conversion of energy to heat. In that process a loss of some energy (to heat, anothe ...
... Friction is the process through which mechanical energy is converted to heat. The breaks in our car heat when we use them and so do our hands when we rub them against each other. Friction can often be an undesirable conversion of energy to heat. In that process a loss of some energy (to heat, anothe ...
g - Cloudfront.net
... 25.000C and carefully add 25.0 mL of 0.500 M HCl, also at 25.000C. After stirring, the final temperature is 27.210C. Calculate qsoln (in J) and DHrxn (in kJ/mol). (Assume the total volume is the sum of the individual volumes and that the final solution has the same density and specfic heat capacity ...
... 25.000C and carefully add 25.0 mL of 0.500 M HCl, also at 25.000C. After stirring, the final temperature is 27.210C. Calculate qsoln (in J) and DHrxn (in kJ/mol). (Assume the total volume is the sum of the individual volumes and that the final solution has the same density and specfic heat capacity ...
Energy
... Thermal Energy • In chemistry we deal primarily with thermal energy. We also encounter light and electrical ...
... Thermal Energy • In chemistry we deal primarily with thermal energy. We also encounter light and electrical ...
Week 4 - Earth & Planetary Sciences
... • If accretion occurs by lots of small impacts, a lot of the energy may be lost to space • If accretion occurs by a few big impacts, all the energy will be deposited in the planet’s interior • Additional energy is released as differentiation occurs – dense iron sinks to centre of planet and releases ...
... • If accretion occurs by lots of small impacts, a lot of the energy may be lost to space • If accretion occurs by a few big impacts, all the energy will be deposited in the planet’s interior • Additional energy is released as differentiation occurs – dense iron sinks to centre of planet and releases ...