Alcohols and Phenols
... Phenols (pKa ~10) are much more acidic than alcohols (pKa ~ 16) because of resonance stabilization of the phenoxide ion Phenols react with NaOH solutions (but alcohols do not), forming salts that are soluble in dilute aqueous solution A phenolic component can be separated from an organic solution by ...
... Phenols (pKa ~10) are much more acidic than alcohols (pKa ~ 16) because of resonance stabilization of the phenoxide ion Phenols react with NaOH solutions (but alcohols do not), forming salts that are soluble in dilute aqueous solution A phenolic component can be separated from an organic solution by ...
Quiz #3 will be concerning Types of Organic Compounds and types
... 7. Ether – any organic compound with the general formula ROR’, where R and R’ are hydrocarbon groups formed by dehydration between two alcohols. 8. Carboxylic acid – an organic compound containing the carboxyl group (-COOH). 9. Alcohol – an organic compound containing one or more hydroxyl (OH) group ...
... 7. Ether – any organic compound with the general formula ROR’, where R and R’ are hydrocarbon groups formed by dehydration between two alcohols. 8. Carboxylic acid – an organic compound containing the carboxyl group (-COOH). 9. Alcohol – an organic compound containing one or more hydroxyl (OH) group ...
OR Practice Problem - HCC Southeast Commons
... sodium amide (NaNH2), and Grignard reagents (RMgX) ...
... sodium amide (NaNH2), and Grignard reagents (RMgX) ...
Organic Chemistry Unit Test! /50
... Thinking and Inquiry (25 marks) 1. Suppose you are working with four unknown compounds in a chemistry laboratory. Your teacher tells you the compounds are ethane, ethanol, ethyl ethanoate, and ethanoic acid. a. Use the table below to identify each unknown compound: (4 marks) ...
... Thinking and Inquiry (25 marks) 1. Suppose you are working with four unknown compounds in a chemistry laboratory. Your teacher tells you the compounds are ethane, ethanol, ethyl ethanoate, and ethanoic acid. a. Use the table below to identify each unknown compound: (4 marks) ...
Physical Properties and Acidity of Carboxylic Acids
... the carbonyl carbon is decreased the acidity of the carboxylic acid will also decrease. Similarly, an increase in its electrophilicity will increase the acidity of the acid. Acetic acid is ten times weaker an acid than formic acid (first two entries in the second row), confirming the electron donati ...
... the carbonyl carbon is decreased the acidity of the carboxylic acid will also decrease. Similarly, an increase in its electrophilicity will increase the acidity of the acid. Acetic acid is ten times weaker an acid than formic acid (first two entries in the second row), confirming the electron donati ...
1. intro notes / aliphatics overview
... *Originally, organic chemistry involved the study of compounds extracted from living organisms. It was believed that organic compounds needed a “vital force” to create them. *However, in 1828 Friedrich Wohler synthesized _______________ (organic) from ________________ chemicals. (Some chemists claim ...
... *Originally, organic chemistry involved the study of compounds extracted from living organisms. It was believed that organic compounds needed a “vital force” to create them. *However, in 1828 Friedrich Wohler synthesized _______________ (organic) from ________________ chemicals. (Some chemists claim ...
Alcohols and Phenols
... positions are much stronger acids The pKa of 2,4,6-trinitrophenol is 0.6, a very strong acid ...
... positions are much stronger acids The pKa of 2,4,6-trinitrophenol is 0.6, a very strong acid ...
3 · Organic Chemistry 3
... There are at least five isomers with the molecular formula, C6H14. Draw and name three of them: ...
... There are at least five isomers with the molecular formula, C6H14. Draw and name three of them: ...
Conceptual Organic Chemistry
... terms of energy difference is to be discussed for all these compounds . Geometrical Isomerism :Requirements for a molecule to show geometrical isomerism, CisTrans and E/ Z notation along with CIP rules for naming geometrical isomers. Optical Isomerism : Optical activity, specific and molar rotation, ...
... terms of energy difference is to be discussed for all these compounds . Geometrical Isomerism :Requirements for a molecule to show geometrical isomerism, CisTrans and E/ Z notation along with CIP rules for naming geometrical isomers. Optical Isomerism : Optical activity, specific and molar rotation, ...
Conceptual Organic Chemistry
... Organic chemistry is the chemistry of carbon compounds and is probably the most active and important field of chemistry, due to its extreme applicability to both, life and industry. Organic chemistry involves few basic principles and many extensions and applications of these principles. After studyi ...
... Organic chemistry is the chemistry of carbon compounds and is probably the most active and important field of chemistry, due to its extreme applicability to both, life and industry. Organic chemistry involves few basic principles and many extensions and applications of these principles. After studyi ...
Conceptual Organic Chemistry
... terms of energy difference is to be discussed for all these compounds . Geometrical Isomerism :Requirements for a molecule to show geometrical isomerism, CisTrans and E/ Z notation along with CIP rules for naming geometrical isomers. Optical Isomerism : Optical activity, specific and molar rotation, ...
... terms of energy difference is to be discussed for all these compounds . Geometrical Isomerism :Requirements for a molecule to show geometrical isomerism, CisTrans and E/ Z notation along with CIP rules for naming geometrical isomers. Optical Isomerism : Optical activity, specific and molar rotation, ...
Lecture 1
... most thoroughly studied and useful reagents. Many of them are commercially available. MeLi is generally handled in ether solution, but RLi compounds with longer chains are soluble in hydrocarbons. Commercial preparation: ...
... most thoroughly studied and useful reagents. Many of them are commercially available. MeLi is generally handled in ether solution, but RLi compounds with longer chains are soluble in hydrocarbons. Commercial preparation: ...
New aniline photocage for carboxylic acids
... removal. On the other hand, for removal of PPGs only iradiation is required (usually UV), which is big advantage in comparison with the standard protecting groups. Three main classes of PPGs described in the literature are based on: 2-nitrobenzyl, carbonyl or benzyl moieties.[4] However, these PPGs ...
... removal. On the other hand, for removal of PPGs only iradiation is required (usually UV), which is big advantage in comparison with the standard protecting groups. Three main classes of PPGs described in the literature are based on: 2-nitrobenzyl, carbonyl or benzyl moieties.[4] However, these PPGs ...
Carbon - Napa Valley College
... Have the same arrangement of atoms but the spatial arrangement of the atoms are different. An example is cis vs trans arrangements across a double bond (cis = large groups are on same side, trans = large groups on opposite side ...
... Have the same arrangement of atoms but the spatial arrangement of the atoms are different. An example is cis vs trans arrangements across a double bond (cis = large groups are on same side, trans = large groups on opposite side ...
Research Poster 36 x 60
... Today only the special chemical compounds undergo testing on biological activity, because the screening is very long-time and expensive process. To these special compounds commonly belong drugs; emulsifiers; test-modifiers; colorants and pigments used in food technology, body-care, etc. But the numb ...
... Today only the special chemical compounds undergo testing on biological activity, because the screening is very long-time and expensive process. To these special compounds commonly belong drugs; emulsifiers; test-modifiers; colorants and pigments used in food technology, body-care, etc. But the numb ...
Organic Families: Summary Chart
... The more C atoms present, the harder it is to break the bonds. The more C atoms, the higher the boiling point. Alkenes and alkynes are more reactive than alkanes due to their double/triple bonds (unsaturated). Presence of OH group makes alcohol more polar than hydrocarbons. Therefore, boiling point ...
... The more C atoms present, the harder it is to break the bonds. The more C atoms, the higher the boiling point. Alkenes and alkynes are more reactive than alkanes due to their double/triple bonds (unsaturated). Presence of OH group makes alcohol more polar than hydrocarbons. Therefore, boiling point ...
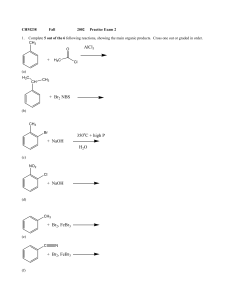
+ NaOH 350 C + high P H2O + H3C AlCl3 + NaOH + Br2, FeBr3
... Starting with toluene, design syntheses, providing the correct reagents for the following transformations. Both processes can be accomplished with 2 steps, but there is more than one correct answer for each. Assume that ortho and para isomers can be separated. OH O CH3 ...
... Starting with toluene, design syntheses, providing the correct reagents for the following transformations. Both processes can be accomplished with 2 steps, but there is more than one correct answer for each. Assume that ortho and para isomers can be separated. OH O CH3 ...
Technical Data Sheet - Lučební závody Kolín
... Synhydrid is a very effective hydridic reduction agent. It plays important role in organic chemistry and is mainly used as a versatile reduction agent for functional groups in organic molecules. Especially it is very effective for reduction of compounds containing carbonyl and carboxyl groups such a ...
... Synhydrid is a very effective hydridic reduction agent. It plays important role in organic chemistry and is mainly used as a versatile reduction agent for functional groups in organic molecules. Especially it is very effective for reduction of compounds containing carbonyl and carboxyl groups such a ...
poly- and heterofunctional compounds
... represented as a sum of properties of separate monofunctional classes. For instance, pyruvic acid (an oxo acid) can be esterified and transformed into derivatives on its carbonyl group. Salicylic and lactic acids (hydroxy acids) form esters in the reaction with alcohols, as well as their hydroxyl gr ...
... represented as a sum of properties of separate monofunctional classes. For instance, pyruvic acid (an oxo acid) can be esterified and transformed into derivatives on its carbonyl group. Salicylic and lactic acids (hydroxy acids) form esters in the reaction with alcohols, as well as their hydroxyl gr ...
Integrated Science Chapter 4 Notes Section 1: Compounds and
... Alkenes have double carbon-carbon bonds ♦ Example: ethylene, C2H4, is the simplest alkene, and has a structure like this: ...
... Alkenes have double carbon-carbon bonds ♦ Example: ethylene, C2H4, is the simplest alkene, and has a structure like this: ...
Synthesis of Benzene Derivatives: Electrophilic Aromatic Substitution
... The initial attack of the electrophile is endothermic because the sp3 carbon generated interrupts the cyclic conjugation. The transition state is not aromatic. The loss of the proton regenerates the sp2 carbon atom and aromaticity is restored. This process is more favored than the nucleophilic trapp ...
... The initial attack of the electrophile is endothermic because the sp3 carbon generated interrupts the cyclic conjugation. The transition state is not aromatic. The loss of the proton regenerates the sp2 carbon atom and aromaticity is restored. This process is more favored than the nucleophilic trapp ...
Phenols

In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group (—OH) bonded directly to an aromatic hydrocarbon group. The simplest of the class is phenol, which is also called carbolic acid C6H5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.Synonyms are arenols or aryl alcohols.Phenolic compounds are synthesized industrially; they also are produced by plants and microorganisms, with variation between and within species.Although similar to alcohols, phenols have unique properties and are not classified as alcohols (since the hydroxyl group is not bonded to a saturated carbon atom). They have higher acidities due to the aromatic ring's tight coupling with the oxygen and a relatively loose bond between the oxygen and hydrogen. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12).Loss of a positive hydrogen ion (H+) from the hydroxyl group of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides, although the term aryloxides is preferred according to the IUPAC Gold Book. Phenols can have two or more hydroxy groups bonded to the aromatic ring(s) in the same molecule. The simplest examples are the three benzenediols, each having two hydroxy groups on a benzene ring.Organisms that synthesize phenolic compounds do so in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research.ref name=Klepacka Some phenols are germicidal and are used in formulating disinfectants. Others possess estrogenic or endocrine disrupting activity.