Ethers - ThinkChemistry
... • How do the melting and boiling points of ethers compare to alcohols? • Why? ...
... • How do the melting and boiling points of ethers compare to alcohols? • Why? ...
Experiment 7
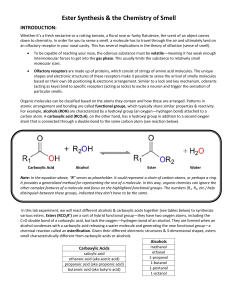
... In this lab experiment, we will react different alcohols & carboxylic acids together (see tables below) to synthesize various esters. Esters (RCO2R’) are a sort of hybrid functional group—they have two oxygen atoms, including the C=O double bond of a carboxylic acid, but lack the oxygen—hydrogen bon ...
... In this lab experiment, we will react different alcohols & carboxylic acids together (see tables below) to synthesize various esters. Esters (RCO2R’) are a sort of hybrid functional group—they have two oxygen atoms, including the C=O double bond of a carboxylic acid, but lack the oxygen—hydrogen bon ...
3.4.3 Corrosives Corrosive materials are those that cause visible
... 3.4.3 Corrosives Corrosive materials are those that cause visible destruction of, or irreversible alterations in, living tissue by chemical action at the site of contact. Corrosives are most commonly acids and alkalis, but many other materials can be severely damaging. Strong oxidizing materials can ...
... 3.4.3 Corrosives Corrosive materials are those that cause visible destruction of, or irreversible alterations in, living tissue by chemical action at the site of contact. Corrosives are most commonly acids and alkalis, but many other materials can be severely damaging. Strong oxidizing materials can ...
Classes of organic acids and bases
... (+) acids & (-) bases Positively charged acids are e- deficient; carbons don’t have an octet • Carbocation is a carbon with a full (+) charge • Highly reactive and seldom seen since the act as intermediates • Carbocations are Lewis acids (hard acids) • React with the first Lewis base they “see” ...
... (+) acids & (-) bases Positively charged acids are e- deficient; carbons don’t have an octet • Carbocation is a carbon with a full (+) charge • Highly reactive and seldom seen since the act as intermediates • Carbocations are Lewis acids (hard acids) • React with the first Lewis base they “see” ...
Introduction to Biodiesel Chemistry
... Organic chemistry is the branch of chemistry that deals with organic compounds. Organic compounds are compounds that (with a few exceptions such as carbon dioxide gas) contain the element carbon. The properties of organic compounds are dependent primarily on the physical structure of the molecules a ...
... Organic chemistry is the branch of chemistry that deals with organic compounds. Organic compounds are compounds that (with a few exceptions such as carbon dioxide gas) contain the element carbon. The properties of organic compounds are dependent primarily on the physical structure of the molecules a ...
CHM1032 Study Guide for Final Exam (including Details for sections... This study guide is only for additional information not covered... Revised December 3, 2014
... 2) Know names and structures of functional groups (scattered in Ch.11-16) as well as hydroxyl group (-OH), and carbonyl group(-C=O). 3) Name and write structural formulas for all alkanes, cycloalkanes, and with halogen groups or alkyl groups. 4) For all other compounds (not in #3 above), be able to ...
... 2) Know names and structures of functional groups (scattered in Ch.11-16) as well as hydroxyl group (-OH), and carbonyl group(-C=O). 3) Name and write structural formulas for all alkanes, cycloalkanes, and with halogen groups or alkyl groups. 4) For all other compounds (not in #3 above), be able to ...
Slide 1
... Given samples of various alcohols and carboxylic acids Establish some of their physical and chemical properties & perform various reactions HO HO ethanol ...
... Given samples of various alcohols and carboxylic acids Establish some of their physical and chemical properties & perform various reactions HO HO ethanol ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 23. a) Explain the mechanism of formation of 2,4-dinitrophenylhydrazone derivative from acetone. b) Compound A with the molecular formula C5H8O2 on reduction forms n-pentane and forms dioxime with hydroxylamine. It gives positive test with Tollen’s reagent and also forms iodoform. Suggest a suitable ...
... 23. a) Explain the mechanism of formation of 2,4-dinitrophenylhydrazone derivative from acetone. b) Compound A with the molecular formula C5H8O2 on reduction forms n-pentane and forms dioxime with hydroxylamine. It gives positive test with Tollen’s reagent and also forms iodoform. Suggest a suitable ...
Nature’s Chemistry
... Alcohols make up a group of organic compounds which contain the -OH group, called the hydroxyl group. The presence of the hydroxyl group in these compounds is indicated by the '-ol' ending of the name of the alcohol. As the alcohols are based on a family of hydrocarbons called alkanes, these alc ...
... Alcohols make up a group of organic compounds which contain the -OH group, called the hydroxyl group. The presence of the hydroxyl group in these compounds is indicated by the '-ol' ending of the name of the alcohol. As the alcohols are based on a family of hydrocarbons called alkanes, these alc ...
Summary of Organic chemistry
... Good solvent for other organic cpnds Ethoxyethane (diethyl ether) used as anaesthetic -ethanoic (acetic) acid produced by fermentation of fruit sugar ethanol ethanoic acid (enzyme req'd) -synthesized from ethyne (acetylene) - most esters have pleasant, fruity flavours -natural and synthetic flav ...
... Good solvent for other organic cpnds Ethoxyethane (diethyl ether) used as anaesthetic -ethanoic (acetic) acid produced by fermentation of fruit sugar ethanol ethanoic acid (enzyme req'd) -synthesized from ethyne (acetylene) - most esters have pleasant, fruity flavours -natural and synthetic flav ...
Carbonyl Compounds
... ethanamide has the highest boiling point because it has the potential t form multiple H-bonds between molecules, all the bonds must be broken before the compound can pass to vapour phase. ethanoic acid, however, has fewer H-bonds compared with ethanamide. Propanone has no possibility forming H-bonds ...
... ethanamide has the highest boiling point because it has the potential t form multiple H-bonds between molecules, all the bonds must be broken before the compound can pass to vapour phase. ethanoic acid, however, has fewer H-bonds compared with ethanamide. Propanone has no possibility forming H-bonds ...
here - University of Queensland
... to seek a funding source. They eventually secured $3.1 million from the Wellcome Trust to launch CO-ADD as a not-for-profit initiative in February 2015. Since then, CO-ADD has received over 110,000 compounds. The program has screened more than 50,000 of these compounds, identified 3000 possible new ...
... to seek a funding source. They eventually secured $3.1 million from the Wellcome Trust to launch CO-ADD as a not-for-profit initiative in February 2015. Since then, CO-ADD has received over 110,000 compounds. The program has screened more than 50,000 of these compounds, identified 3000 possible new ...
Synthesis of Esters Problem: Produce an ester that smells
... Combinatorial chemistry is a technology for producing large numbers of diverse, but related compounds. The pharmaceutical, agrochemical, and biotechnology industries use combinatorial chemistry to reduce the time and cost associated with the discovery of new drugs, pesticides, and peptides. Instead ...
... Combinatorial chemistry is a technology for producing large numbers of diverse, but related compounds. The pharmaceutical, agrochemical, and biotechnology industries use combinatorial chemistry to reduce the time and cost associated with the discovery of new drugs, pesticides, and peptides. Instead ...
03 AP Bio Carbon and the Molecular Diversity of Life
... system was now in the form of organic compounds. ...
... system was now in the form of organic compounds. ...
S04 Organic Molecules and Functional Groups
... • Distinctive properties of organic molecules depend on the carbon skeleton and on the molecular components attached to it • A number of characteristic groups can replace the hydrogens attached to skeletons of organic molecules ...
... • Distinctive properties of organic molecules depend on the carbon skeleton and on the molecular components attached to it • A number of characteristic groups can replace the hydrogens attached to skeletons of organic molecules ...
TT T p
... is saidto haveoccurred.The products,calledhalogen derivatives,are named usihg a standardsystem. The suffix comes from the name of the straieht-chain alkane with the same number of carbon atoms.The prefix shows which atoms or functional'groups have been added and the carbon atom in the straight chain ...
... is saidto haveoccurred.The products,calledhalogen derivatives,are named usihg a standardsystem. The suffix comes from the name of the straieht-chain alkane with the same number of carbon atoms.The prefix shows which atoms or functional'groups have been added and the carbon atom in the straight chain ...
GCE A level 1094/01 CHEMISTRY CH4
... Phenol was isolated from coal tar in 1835 and its original name was carbolic acid. It is a weak acid, between carboxylic acids and alcohols in strength. In 1865 the English surgeon Joseph Lister pioneered the use of phenol as the first surgical antiseptic and by the beginning of the 20th century phe ...
... Phenol was isolated from coal tar in 1835 and its original name was carbolic acid. It is a weak acid, between carboxylic acids and alcohols in strength. In 1865 the English surgeon Joseph Lister pioneered the use of phenol as the first surgical antiseptic and by the beginning of the 20th century phe ...
Interactive comment on “Observations of oxidation products
... But, if the conclusions from this study are valid, than we have to assume that possibly 90 percent of terpene emissions were overlooked by all of these studies? These thoughts make me wonder how solid the presented experiments, interpretations and conclusions are. Taking a closer look, I do see quit ...
... But, if the conclusions from this study are valid, than we have to assume that possibly 90 percent of terpene emissions were overlooked by all of these studies? These thoughts make me wonder how solid the presented experiments, interpretations and conclusions are. Taking a closer look, I do see quit ...
Functional Groups - Waterford Public Schools
... • Organic compounds with one or more hydroxyl groups, -OH • General formula: CnH2n+1OH • Three classes of alcohols: ...
... • Organic compounds with one or more hydroxyl groups, -OH • General formula: CnH2n+1OH • Three classes of alcohols: ...
Document
... cells in organisms. They are responsible for everything from the storage of energy to support structures within a cell system. The chemical elements carbon, hydrogen, oxygen, phosphorus, sulfur and nitrogen in different combinations, make up each of the molecules. How these elements are arranged dic ...
... cells in organisms. They are responsible for everything from the storage of energy to support structures within a cell system. The chemical elements carbon, hydrogen, oxygen, phosphorus, sulfur and nitrogen in different combinations, make up each of the molecules. How these elements are arranged dic ...
Organic and Biochemical Molecules 1. Compounds composed of
... an acceptor. Thus, the carboxylic acid (with greater areas for H-bonding) would be more soluble in water. The methanol molecule would be the next in line for most soluble. The smaller the “R-chain” the greater the extent of solubility. Remember that because the Rchain is nonpolar, it, on its own, w ...
... an acceptor. Thus, the carboxylic acid (with greater areas for H-bonding) would be more soluble in water. The methanol molecule would be the next in line for most soluble. The smaller the “R-chain” the greater the extent of solubility. Remember that because the Rchain is nonpolar, it, on its own, w ...
Organic Chemistry Questions
... Dimethyl ether, H3C–O–CH3, is not very soluble in water. Draw a structural isomer of dimethyl ether that is much more soluble in water and explain the basis of its increased water solubility. ...
... Dimethyl ether, H3C–O–CH3, is not very soluble in water. Draw a structural isomer of dimethyl ether that is much more soluble in water and explain the basis of its increased water solubility. ...
CHEMISTRY 3.5 Paper 1 Describe the structure and reactions of
... acidic conditions, the amino acid forms an ion that will move towards one electrode. In basic conditions, it forms another ion that will move towards the other electrode. Explain how the conditions described above give rise to two ions that will move towards the two different electrodes and state wh ...
... acidic conditions, the amino acid forms an ion that will move towards one electrode. In basic conditions, it forms another ion that will move towards the other electrode. Explain how the conditions described above give rise to two ions that will move towards the two different electrodes and state wh ...
Phenols

In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group (—OH) bonded directly to an aromatic hydrocarbon group. The simplest of the class is phenol, which is also called carbolic acid C6H5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.Synonyms are arenols or aryl alcohols.Phenolic compounds are synthesized industrially; they also are produced by plants and microorganisms, with variation between and within species.Although similar to alcohols, phenols have unique properties and are not classified as alcohols (since the hydroxyl group is not bonded to a saturated carbon atom). They have higher acidities due to the aromatic ring's tight coupling with the oxygen and a relatively loose bond between the oxygen and hydrogen. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12).Loss of a positive hydrogen ion (H+) from the hydroxyl group of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides, although the term aryloxides is preferred according to the IUPAC Gold Book. Phenols can have two or more hydroxy groups bonded to the aromatic ring(s) in the same molecule. The simplest examples are the three benzenediols, each having two hydroxy groups on a benzene ring.Organisms that synthesize phenolic compounds do so in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research.ref name=Klepacka Some phenols are germicidal and are used in formulating disinfectants. Others possess estrogenic or endocrine disrupting activity.