Potentiometry and NMR studies of 1,5,9
... average value (log K I = 12.8) is quite similar to the first protonation constant of [12]ane-N, (log K1 = 12.616). The protonation constants of DOTRA3- obtained by spectrophotometry and pH potentiometry are shown in Table 11, where the protonation constants of [ 121ane-N,, as well as [9]ane-N3 and N ...
... average value (log K I = 12.8) is quite similar to the first protonation constant of [12]ane-N, (log K1 = 12.616). The protonation constants of DOTRA3- obtained by spectrophotometry and pH potentiometry are shown in Table 11, where the protonation constants of [ 121ane-N,, as well as [9]ane-N3 and N ...
Synthesis of hydroborate compounds as potential chemical vapor
... diboride (MgB2), which becomes superconducting below ca. 40 K,7 is potentially useful for the fabrication of superconductor-based integrated circuits. For many transition metal boride phases, it is not possible to use single source CVD precursors to grow films because no precursor of stoichiometry M ...
... diboride (MgB2), which becomes superconducting below ca. 40 K,7 is potentially useful for the fabrication of superconductor-based integrated circuits. For many transition metal boride phases, it is not possible to use single source CVD precursors to grow films because no precursor of stoichiometry M ...
Chelate chemistry for molecular imaging Ligands versus Chelates
... 2) M+ (softer) < M2+ (intermediate) < M3+ (harder) V2+ (79 pm) >> V3+ (64 pm) >> V4+ (53 pm) ...
... 2) M+ (softer) < M2+ (intermediate) < M3+ (harder) V2+ (79 pm) >> V3+ (64 pm) >> V4+ (53 pm) ...
The complex in biological systems Plan 1. Definition of complex
... domestic sphere, form outer sphere. The charge computer. Jonah determined by simple algebraic amounts of charges ions it contains. A significant contribution to the development of the theory of coordination made Chuhayev, Kurnikov, Yatsymirskyy. An important feature of the ligands is their capacity ...
... domestic sphere, form outer sphere. The charge computer. Jonah determined by simple algebraic amounts of charges ions it contains. A significant contribution to the development of the theory of coordination made Chuhayev, Kurnikov, Yatsymirskyy. An important feature of the ligands is their capacity ...
Chapter 9 ( Cyclopentadienyl)
... This in spite of the fact that the metal’s covalent radius is decreasing as one goes from Fe to Ni. Problem: Explain why the Fe-C distance lengthens for [Cp2Fe]+, while the Co-C distance shortens for [Cp2Co]+. ...
... This in spite of the fact that the metal’s covalent radius is decreasing as one goes from Fe to Ni. Problem: Explain why the Fe-C distance lengthens for [Cp2Fe]+, while the Co-C distance shortens for [Cp2Co]+. ...
Thermodynamics and Further Inorganic Chemistry
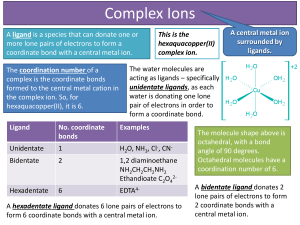
... change of co-ordination number. • The Cl− ligand is larger than these uncharged ligands and that ligand exchange can involve a change of co-ordination number. • Substitution of unidentate ligands (ligands which forms one bond with the central metal) with a bidentate or a multidentate ligand leads to ...
... change of co-ordination number. • The Cl− ligand is larger than these uncharged ligands and that ligand exchange can involve a change of co-ordination number. • Substitution of unidentate ligands (ligands which forms one bond with the central metal) with a bidentate or a multidentate ligand leads to ...
Lab 11
... coordination number of a metal ion is the number of bonds it can form with ligands – typically 1, 2, 3, 4, 5, or 6. The coordination number of Fe3+ is 6. ...
... coordination number of a metal ion is the number of bonds it can form with ligands – typically 1, 2, 3, 4, 5, or 6. The coordination number of Fe3+ is 6. ...
Metal Sequestration (English version)
... properties of certain phosphates. With the introduction of the amino carboxylic acids and particularly ethylene diamino tetracarboxylic acid (EDTA) the term has become increasingly associated with the products having similar action. Sequestration is the suppression of a property or reaction of a met ...
... properties of certain phosphates. With the introduction of the amino carboxylic acids and particularly ethylene diamino tetracarboxylic acid (EDTA) the term has become increasingly associated with the products having similar action. Sequestration is the suppression of a property or reaction of a met ...
Citation Exercise.CH..
... Transition metal complexes are typically very colorful because the differences between the d-orbital energy levels match the energy of visible radiation. Cobalt(II) is a d7 transition metal that changes color when involved in the following reaction Co(H2O)62+(aq) + 4 Cl-(aq) CoCl42-(aq) + 6 H2O(l) ...
... Transition metal complexes are typically very colorful because the differences between the d-orbital energy levels match the energy of visible radiation. Cobalt(II) is a d7 transition metal that changes color when involved in the following reaction Co(H2O)62+(aq) + 4 Cl-(aq) CoCl42-(aq) + 6 H2O(l) ...
23.2 - Transition-Metal Complexes 23.3
... present Optical Isomerism - These are enantiomers, which are mirror images that cannot be super-imposed on one another Tetrahedrals cannot form geometric isomers since it's the same when viewed from any side Chelating agents can't span across the coordinate sphere, which limits the number of isomers ...
... present Optical Isomerism - These are enantiomers, which are mirror images that cannot be super-imposed on one another Tetrahedrals cannot form geometric isomers since it's the same when viewed from any side Chelating agents can't span across the coordinate sphere, which limits the number of isomers ...
FACTORS AFFECT
... VALENCE BOND THEORY This theory was developed by Linus Pauling. According to this theory the bonding in metal complexes arises when a filled ligand orbital containing a pair of electrons overlaps with a vacant hybrid orbital on the metal atom. The VBT assumes the bonding between the metal atom an ...
... VALENCE BOND THEORY This theory was developed by Linus Pauling. According to this theory the bonding in metal complexes arises when a filled ligand orbital containing a pair of electrons overlaps with a vacant hybrid orbital on the metal atom. The VBT assumes the bonding between the metal atom an ...
The Solubility of Calcium Sulfate
... Multidentate Ligand: a ligand attaches to a metal ion through more than one ligand atoms: chelating ligand. ...
... Multidentate Ligand: a ligand attaches to a metal ion through more than one ligand atoms: chelating ligand. ...
Investigation of Nickel and Copper Coordination Complexes
... Typically, as ligand is added to the solution of metal ion, ML is formed first. As the addition of ligand is continued, the ML2 concentration rises, while the ML concentration drops. Then ML3 becomes dominant with ML and ML2 becoming unimportant. This process continues until the highest complex, ML6 ...
... Typically, as ligand is added to the solution of metal ion, ML is formed first. As the addition of ligand is continued, the ML2 concentration rises, while the ML concentration drops. Then ML3 becomes dominant with ML and ML2 becoming unimportant. This process continues until the highest complex, ML6 ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... b) Discuss the electron transfer reaction in organometallic compound. ...
... b) Discuss the electron transfer reaction in organometallic compound. ...
Transition Metals & Complex ions
... Formulae Questions 1. State the formula & charge of each of these complex ions: a) One copper (II) ion & four chloride ions b) One iron (III) ion & five water molecules & a chloride ion. c) One copper (II) ion & four ammonia molecules & two hydroxide ions d) One cobalt (III) ion & four hydroxide io ...
... Formulae Questions 1. State the formula & charge of each of these complex ions: a) One copper (II) ion & four chloride ions b) One iron (III) ion & five water molecules & a chloride ion. c) One copper (II) ion & four ammonia molecules & two hydroxide ions d) One cobalt (III) ion & four hydroxide io ...