Transition Metals - wellswaysciences
... ethanedioate ions form one dative bond from each N in the molecule so are bidentate. • These can form octahedral complexes with cis-trans isomers as long as 2 bidentate ligands are present with 2 monodentate ligands, e.g. [CoCl2(en)2] or [Cr(C2O4)2(H2O)2]• EDTA is a chelating hexadentate ligand – ch ...
... ethanedioate ions form one dative bond from each N in the molecule so are bidentate. • These can form octahedral complexes with cis-trans isomers as long as 2 bidentate ligands are present with 2 monodentate ligands, e.g. [CoCl2(en)2] or [Cr(C2O4)2(H2O)2]• EDTA is a chelating hexadentate ligand – ch ...
Assignment 3
... equilibrates with respect to gas-liquid exchange? Assume that redox reactions are kinetically inhibited. ...
... equilibrates with respect to gas-liquid exchange? Assume that redox reactions are kinetically inhibited. ...
InorgCh14.1
... Ligand (CO) Substitution is important for synthesis of new complexes a) Rate is independent of incoming ligand = D mechanism (for most) Ni(CO)4 Ni(CO)3 18e- to 16e- (slow) Ni(CO)3 + L Ni(CO)3L 16e- to 18e- (fast) b) ...
... Ligand (CO) Substitution is important for synthesis of new complexes a) Rate is independent of incoming ligand = D mechanism (for most) Ni(CO)4 Ni(CO)3 18e- to 16e- (slow) Ni(CO)3 + L Ni(CO)3L 16e- to 18e- (fast) b) ...
Downloadmela
... b. The epr spectrum of a high spin Mn(II) complex, doped onto a diamagnetic host, consists of 30 epr lines when there is no hyperfine splitting by the ligand. Interpret the spectrum with the help of a qualitative hyperfine splitting diagram. (7) 26a. -Donation by ligands lowers 10Dq values whereas ...
... b. The epr spectrum of a high spin Mn(II) complex, doped onto a diamagnetic host, consists of 30 epr lines when there is no hyperfine splitting by the ligand. Interpret the spectrum with the help of a qualitative hyperfine splitting diagram. (7) 26a. -Donation by ligands lowers 10Dq values whereas ...
Colors & How We Preceive it
... ligands explains nicely the origin of color and magnetism for these compounds. There is evidence to suggest that the metal-ligand bond has covalent character which explains why these complexes are very stable. Molecular Orbital Theory can also be used to describe the bonding scheme in these complexe ...
... ligands explains nicely the origin of color and magnetism for these compounds. There is evidence to suggest that the metal-ligand bond has covalent character which explains why these complexes are very stable. Molecular Orbital Theory can also be used to describe the bonding scheme in these complexe ...
Solutions to Problem Set #9
... 19.10 [Cr(NH3)6]Cl3 [Co(H2NCH2CH2NH2)2(OH2)(NCS)]NO3 K2[Ni(CN)4] [Co(H 2NCH2CH2NH2)3]I3 19.14 [Fe(CN)6]3- will be low spin because cyanide is a strong field ligand which in itself is enough, but this is also a trivalent metal ion, which also serves to increase the crystal field splitting (relative t ...
... 19.10 [Cr(NH3)6]Cl3 [Co(H2NCH2CH2NH2)2(OH2)(NCS)]NO3 K2[Ni(CN)4] [Co(H 2NCH2CH2NH2)3]I3 19.14 [Fe(CN)6]3- will be low spin because cyanide is a strong field ligand which in itself is enough, but this is also a trivalent metal ion, which also serves to increase the crystal field splitting (relative t ...
Transition Metal Chemistry
... oxidation states, there needs to be greater packets of (single) energy. Note: removal of successive e-'s ...
... oxidation states, there needs to be greater packets of (single) energy. Note: removal of successive e-'s ...
A1982NU66300001
... These were exciting times at University College London, with many new complexes being produced each week with the enthusiastic encouragementof Ron Nyholm. It was clear that there was a real need, in order to support this synthetic programme, for a rapid spectroscopic method for telling the likely ox ...
... These were exciting times at University College London, with many new complexes being produced each week with the enthusiastic encouragementof Ron Nyholm. It was clear that there was a real need, in order to support this synthetic programme, for a rapid spectroscopic method for telling the likely ox ...
Periodicity (AHL)
... Magnetic nature of some TM The magnetism of a TM is determined by the number of unpaired electrons it has in its configuration. In complexes, the coordination and structure are also factors. Because e- spin, they create a magnetic field and act like tiny magnets. When they are paired, they cancel e ...
... Magnetic nature of some TM The magnetism of a TM is determined by the number of unpaired electrons it has in its configuration. In complexes, the coordination and structure are also factors. Because e- spin, they create a magnetic field and act like tiny magnets. When they are paired, they cancel e ...
InorgCh11.1
... 1. Electron-Electron Interactions complicate the spectra of multi-electron species 2. Example: [V(H2O)6]3+ is an octahedral d2 metal ion [V(H2O)6]3+ ...
... 1. Electron-Electron Interactions complicate the spectra of multi-electron species 2. Example: [V(H2O)6]3+ is an octahedral d2 metal ion [V(H2O)6]3+ ...
(over) Candidate: Agozie Nnaemeka Oyeamalu For the degree of
... molecular and geometric characteristics of molecules. In this study, we were able to solve structures of new metallacarboranes and other complexes via X-ray crystallography. This resulted in specifically two crystallographically independent molecules in the unit cell complex of the metallacarborane ...
... molecular and geometric characteristics of molecules. In this study, we were able to solve structures of new metallacarboranes and other complexes via X-ray crystallography. This resulted in specifically two crystallographically independent molecules in the unit cell complex of the metallacarborane ...
TM shape and colour
... SPLITTING OF 3d ORBITALS Placing ligands around a central ion causes the energies of the d orbitals to change Some of the d orbitals gain energy and some lose energy In an octahedral complex, two (z2 and x2-y2) go higher and three go lower In a tetrahedral complex, three (xy, xz and yz) go higher a ...
... SPLITTING OF 3d ORBITALS Placing ligands around a central ion causes the energies of the d orbitals to change Some of the d orbitals gain energy and some lose energy In an octahedral complex, two (z2 and x2-y2) go higher and three go lower In a tetrahedral complex, three (xy, xz and yz) go higher a ...
coordination chemistry notes
... contribution from dispersion forces between polarisable atoms. In some cases, πbackbonding may also be important. It is important to make the distinction between Hardness and Softness and Acid/Base Strength. Hardness/Softness has already been defined as the special stability of hardhard and soft-sof ...
... contribution from dispersion forces between polarisable atoms. In some cases, πbackbonding may also be important. It is important to make the distinction between Hardness and Softness and Acid/Base Strength. Hardness/Softness has already been defined as the special stability of hardhard and soft-sof ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 3. In the presence of moderate to strong field ligands Co(II) has a strong tendency to get oxidized to the Co(III) state in aqueous solution. Give reason. 4. The CN stretching vibration in cyano complexes occur at higher energy than that of free cyanide ion. Explain. 5. Differentiate trans-effect an ...
... 3. In the presence of moderate to strong field ligands Co(II) has a strong tendency to get oxidized to the Co(III) state in aqueous solution. Give reason. 4. The CN stretching vibration in cyano complexes occur at higher energy than that of free cyanide ion. Explain. 5. Differentiate trans-effect an ...
i PREFACE The ability of Schiff bases to form complexes with metal
... to the >C=N- group. Besides the ability to form complexes with metals, Schiff bases and their metal complexes have innumerable applications in many fields namely medicine, pharmacy, agriculture, photography, catalysis, polymer technology, paints and pigments and industries such as textiles, glass, a ...
... to the >C=N- group. Besides the ability to form complexes with metals, Schiff bases and their metal complexes have innumerable applications in many fields namely medicine, pharmacy, agriculture, photography, catalysis, polymer technology, paints and pigments and industries such as textiles, glass, a ...
Crystal Field Theory www.AssignmentPoint.com Crystal Field
... orbitals will be dxy, dxz and dyz - opposite to the octahedral case. Furthermore, since the ligand electrons in tetrahedral symmetry are not oriented directly towards the d-orbitals, the energy splitting will be lower than in the octahedral ...
... orbitals will be dxy, dxz and dyz - opposite to the octahedral case. Furthermore, since the ligand electrons in tetrahedral symmetry are not oriented directly towards the d-orbitals, the energy splitting will be lower than in the octahedral ...
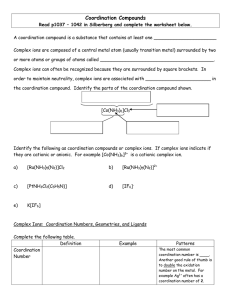
Coordination Compounds
... Main Postulates of Werner’s Theory: i) Metals show two types of valences in coordination compounds- Primary & Secondary. ii) The Primary valences are normally ionizable and are satisfied by negative ions. iii) The Secondary valences are non ionizable and are satisfied by neutral molecules or negativ ...
... Main Postulates of Werner’s Theory: i) Metals show two types of valences in coordination compounds- Primary & Secondary. ii) The Primary valences are normally ionizable and are satisfied by negative ions. iii) The Secondary valences are non ionizable and are satisfied by neutral molecules or negativ ...
transition metals
... 6.) Using the ideas of Lewis and resonance structures, determine which of the following ligands can participate in linkage isomerism (meaning can they cause a compound to be a linkage isomer). DRAW the resonance structures in order to explain! Remember that a linkage isomer can bond to the metal ion ...
... 6.) Using the ideas of Lewis and resonance structures, determine which of the following ligands can participate in linkage isomerism (meaning can they cause a compound to be a linkage isomer). DRAW the resonance structures in order to explain! Remember that a linkage isomer can bond to the metal ion ...