transition metal - Compound Interest
... the metal atom in orbitals. Transition metals, unlike other metals, have partially filled d orbitals, which can hold up to 10 electrons. When ligands are present, some d orbitals become higher in energy than before, and some become lower. Electrons can then move between these higher and lower d orbi ...
... the metal atom in orbitals. Transition metals, unlike other metals, have partially filled d orbitals, which can hold up to 10 electrons. When ligands are present, some d orbitals become higher in energy than before, and some become lower. Electrons can then move between these higher and lower d orbi ...
STABILITETEN AF KOORDINATIONSFORBINDELSER
... αn is a convenient entity when the relative distribution of species are plotted, using the estimated βn ‘s.. Example: Cadmium ions form complexes with ammonia as the ligand with up to 6 ligands coordinated. Logβn values are 2.66, 4.76, 6.18, 7.11, 6.84, 4.4, respectively. The numbers reveal that the ...
... αn is a convenient entity when the relative distribution of species are plotted, using the estimated βn ‘s.. Example: Cadmium ions form complexes with ammonia as the ligand with up to 6 ligands coordinated. Logβn values are 2.66, 4.76, 6.18, 7.11, 6.84, 4.4, respectively. The numbers reveal that the ...
Design and Synthesis of a New Chelate for the Uranyl Ion
... Biological Chemistry Department, College of Judea and Samaria, Ariel, Israel The hard acid, large uranyl cation is expected to form stable complexes with diamino tetradentate dianionic open chain ligands. As the two axial position of the dioxo cation are occupied it was decided to design a flexible ...
... Biological Chemistry Department, College of Judea and Samaria, Ariel, Israel The hard acid, large uranyl cation is expected to form stable complexes with diamino tetradentate dianionic open chain ligands. As the two axial position of the dioxo cation are occupied it was decided to design a flexible ...
IOSR Journal of Applied Chemistry (IOSR-JAC) e-ISSN: 2278-5736.
... Lactum form, α-hydroxyl and ϒ- hydroxyl group ionizes in alkaline medium corresponding to two pK values. It has been concluded that Cu(II) and Co(II) binds with thymine through ring nitrogen and carbonyl oxygen whereas in Ni(II) complex bonding occurs from both the ring nitrogen. In Zn(II) complex a ...
... Lactum form, α-hydroxyl and ϒ- hydroxyl group ionizes in alkaline medium corresponding to two pK values. It has been concluded that Cu(II) and Co(II) binds with thymine through ring nitrogen and carbonyl oxygen whereas in Ni(II) complex bonding occurs from both the ring nitrogen. In Zn(II) complex a ...
Powerpoint - mvhs
... CoCl3*6NH3 while the purple compound only has 5 ammonia molecules in the coordinated compound. As shown in the ball-and-stick model, the chlorides serve as counter ions to the cobalt/ammonia coordiation complex in the orange compound, while one of the ammonia molecules is replaced by Cl in the purpl ...
... CoCl3*6NH3 while the purple compound only has 5 ammonia molecules in the coordinated compound. As shown in the ball-and-stick model, the chlorides serve as counter ions to the cobalt/ammonia coordiation complex in the orange compound, while one of the ammonia molecules is replaced by Cl in the purpl ...
IOSR Journal of Applied Chemistry (IOSR-JAC)
... bring the reacting molecules together to give activated complex [g] but also polarised electrons from the ligands is indicated towards the metal. The relation between stability and bsicity of the ligands is indicated by the formation constant and free energy change value. Bulkier group increases the ...
... bring the reacting molecules together to give activated complex [g] but also polarised electrons from the ligands is indicated towards the metal. The relation between stability and bsicity of the ligands is indicated by the formation constant and free energy change value. Bulkier group increases the ...
hard acid
... The word ligand is derived from the Latin verb ‘ligare’ meaning ‘to bind’. In a coordination complex, a central atom or ion is coordinated by one or more molecules or ions (ligands) which act as Lewis bases, forming coordinate bonds with the central atom or ion; the latter acts as a Lewis acid. Atom ...
... The word ligand is derived from the Latin verb ‘ligare’ meaning ‘to bind’. In a coordination complex, a central atom or ion is coordinated by one or more molecules or ions (ligands) which act as Lewis bases, forming coordinate bonds with the central atom or ion; the latter acts as a Lewis acid. Atom ...
Review Quiz 7 - ltcconline.net
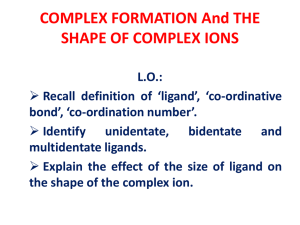
... A complex ion and counterion with no net charge. Complex ion: Charged species consisting of metal surrounded by ligands. Counterion: Anion/Cation that balance the charge on the complex ion in a coordination compound. Coordination #: Number of bonds formed between the metal ion and ligands (usually 4 ...
... A complex ion and counterion with no net charge. Complex ion: Charged species consisting of metal surrounded by ligands. Counterion: Anion/Cation that balance the charge on the complex ion in a coordination compound. Coordination #: Number of bonds formed between the metal ion and ligands (usually 4 ...
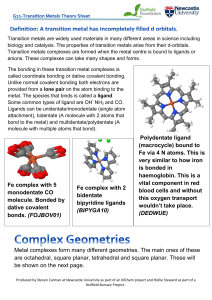
Polydentate ligand (macrocycle) bound to Fe via 4 N atoms. This is
... wouldn’t take place. (DEDWUE) ...
... wouldn’t take place. (DEDWUE) ...
Chapter 24: Transition Metals Coordination Compounds Part 2
... • We see the color which is NOT absorbed, but which was reflected or transmitted. • We see the complementary color of what was absorbed. ...
... • We see the color which is NOT absorbed, but which was reflected or transmitted. • We see the complementary color of what was absorbed. ...
Complex Ions and Free Energy
... form between metal ions and ligands. Furthermore, I can determine the coordination number for a coordination complex • LT 8.7 – I can calculate the formation constant for complex ions and relate that to the Ksp for a slightly soluble compound. • LT 8.8 – I can calculate the free energy of a chemical ...
... form between metal ions and ligands. Furthermore, I can determine the coordination number for a coordination complex • LT 8.7 – I can calculate the formation constant for complex ions and relate that to the Ksp for a slightly soluble compound. • LT 8.8 – I can calculate the free energy of a chemical ...
Scientific abstract
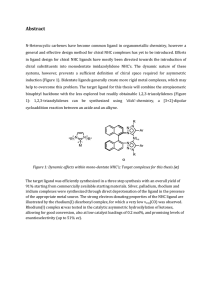
... chiral substituents into monodentate imidazolylidene NHC’s. The dynamic nature of these systems, however, prevents a sufficient definition of chiral space required for asymmetric induction (Figure 1). Bidentate ligands generally create more rigid metal complexes, which may help to overcome this prob ...
... chiral substituents into monodentate imidazolylidene NHC’s. The dynamic nature of these systems, however, prevents a sufficient definition of chiral space required for asymmetric induction (Figure 1). Bidentate ligands generally create more rigid metal complexes, which may help to overcome this prob ...
Stability constants for complexes of the siderophore
... • Pu(IV)-DFO complexes form from any oxidation state of Pu (III, IV, V, or VI) • Above pH=6, Pu (VI) is reduced irreversibly to Pu(IV), and reduction is assisted by higher DFO concentrations • Surprisingly, Pu(IV)-DFO complex is still reactive, since Pu(VI) will be reduced even though the DFO is alr ...
... • Pu(IV)-DFO complexes form from any oxidation state of Pu (III, IV, V, or VI) • Above pH=6, Pu (VI) is reduced irreversibly to Pu(IV), and reduction is assisted by higher DFO concentrations • Surprisingly, Pu(IV)-DFO complex is still reactive, since Pu(VI) will be reduced even though the DFO is alr ...
Hydrogeochemistry
... Toxicity depends on activity and complexes not total concentrations E.g., CH3Hg+ and Cu2+ are toxic to fish other complexes, e.g., CuCO3o are not ...
... Toxicity depends on activity and complexes not total concentrations E.g., CH3Hg+ and Cu2+ are toxic to fish other complexes, e.g., CuCO3o are not ...