Chemistry of Coordination Compounds
... ammonia molecules in the coordinated compound. As shown in the ball-and-stick model, the chlorides serve as counter ions to the cobalt/ammonia coordiation complex in the orange compound, while one of the ammonia molecules is replaced by Cl in the purple compound. In both cases, the coordination geom ...
... ammonia molecules in the coordinated compound. As shown in the ball-and-stick model, the chlorides serve as counter ions to the cobalt/ammonia coordiation complex in the orange compound, while one of the ammonia molecules is replaced by Cl in the purple compound. In both cases, the coordination geom ...
SC-Database - u
... any of over 130 metal ions (e.g. Cu+, Cu++, Cu+++) alkali metal ions alkaline earth metal ions lanthanide ions alternatively compare constants for 2 metal ions ...
... any of over 130 metal ions (e.g. Cu+, Cu++, Cu+++) alkali metal ions alkaline earth metal ions lanthanide ions alternatively compare constants for 2 metal ions ...
CHAPTER 11
... formation. The first four pK values apply to carboxyl protons, and the last two are for the ...
... formation. The first four pK values apply to carboxyl protons, and the last two are for the ...
thermodynamic study of solvation and complex formation in system
... characteristics of disolution Cu(NO3)2 salt, that is caused by formation of complexes Cu(II) with β-alanine and also dissociation of amino acid during complexation. The measured enthalpies of on complex formation are negative at all range water-ethanol mixtures. At the same time process of dissociat ...
... characteristics of disolution Cu(NO3)2 salt, that is caused by formation of complexes Cu(II) with β-alanine and also dissociation of amino acid during complexation. The measured enthalpies of on complex formation are negative at all range water-ethanol mixtures. At the same time process of dissociat ...
Chapter 24
... both due to the presence of Cr+3 ions – the difference lies in the crystal hosting the ion In rubies, some Al+3 ions in the Al2O3 are replaced by Cr+3 ions. In emeralds, some Al+3 ions in the Be3Al2(SiO3)6 are replaced by Cr+3 ions. ...
... both due to the presence of Cr+3 ions – the difference lies in the crystal hosting the ion In rubies, some Al+3 ions in the Al2O3 are replaced by Cr+3 ions. In emeralds, some Al+3 ions in the Be3Al2(SiO3)6 are replaced by Cr+3 ions. ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034 Part-A
... Answer any eight questions. Each question carries five marks. ...
... Answer any eight questions. Each question carries five marks. ...
A1988Q406500001
... 2~ series that exhibited striking similari- frequent citation no doubt is the absence of a ligand ties to the well-known spectrochemical ligand any more recent general review of V02+. series that had generally been generated from Later work by many others with V02+, unoptical spectral data. It occur ...
... 2~ series that exhibited striking similari- frequent citation no doubt is the absence of a ligand ties to the well-known spectrochemical ligand any more recent general review of V02+. series that had generally been generated from Later work by many others with V02+, unoptical spectral data. It occur ...
A1988Q406700001
... 2~ series that exhibited striking similari- frequent citation no doubt is the absence of a ligand ties to the well-known spectrochemical ligand any more recent general review of V02+. series that had generally been generated from Later work by many others with V02+, unoptical spectral data. It occur ...
... 2~ series that exhibited striking similari- frequent citation no doubt is the absence of a ligand ties to the well-known spectrochemical ligand any more recent general review of V02+. series that had generally been generated from Later work by many others with V02+, unoptical spectral data. It occur ...
Electrochemical, in-vitro in-vivo study of Co (II)-ofloxacin complex Deepa Thakur ,Jyotsna Mishra,
... and diffusion controlled Polarographic wave which has revealed by the log plot slope and the plots of i d versus √h effective hight of mercury column respectively on the gradual addition of the ligand the E 1/2 of the metal shifted to more electronegative values indicating the formulation of Co (II) ...
... and diffusion controlled Polarographic wave which has revealed by the log plot slope and the plots of i d versus √h effective hight of mercury column respectively on the gradual addition of the ligand the E 1/2 of the metal shifted to more electronegative values indicating the formulation of Co (II) ...
Studies of stability constant on ternary chelates of bivalent
... complexes. In all the ternary systems, distinct inflections were observed in the titration curves, indicating the formation of chelates. Formation of ternary complexes was further confirmed from the non-superimposible nature of theoretical composite curves on the experimental curve in the region of ...
... complexes. In all the ternary systems, distinct inflections were observed in the titration curves, indicating the formation of chelates. Formation of ternary complexes was further confirmed from the non-superimposible nature of theoretical composite curves on the experimental curve in the region of ...
Tris-2,2’-bipyridine Complexes of Iron(II) and Ruthenium (II): Synthesis, Spectroscopy and Electrochemistry
... You will probe the redox behavior of [Ru(bpy)3]2+ and [Fe(bpy)3]2+. Cyclic Voltammetry (CV) will be used to make the measurements. Make up 50 mL of acetonitrile/0.1 M TEABF4 (tetraethylammonium tetrafluoroborate), set up the electrochemical cell and bubble the solution with argon or nitrogen for app ...
... You will probe the redox behavior of [Ru(bpy)3]2+ and [Fe(bpy)3]2+. Cyclic Voltammetry (CV) will be used to make the measurements. Make up 50 mL of acetonitrile/0.1 M TEABF4 (tetraethylammonium tetrafluoroborate), set up the electrochemical cell and bubble the solution with argon or nitrogen for app ...
Complex Ions
... What is a Complex Ion? A property of transition metals is their ability to form complex ions Complex ion:Central metal ion is surrounded by ligands Ligand: Molecule / ion which donates a pair of electrons forming a coordinate bond (dative bond) Coordinate bond: One of the bonded atoms has provided ...
... What is a Complex Ion? A property of transition metals is their ability to form complex ions Complex ion:Central metal ion is surrounded by ligands Ligand: Molecule / ion which donates a pair of electrons forming a coordinate bond (dative bond) Coordinate bond: One of the bonded atoms has provided ...
Bidentate & multidentate ligands File
... a) Explain the term “bidentate ligand”. b) What is the coordination number of the [Fe(C2O4)3]3complex. c) Use your answer to part (b) to suggest what shape the [Fe(C2O4)3]3- complex is and draw it. ...
... a) Explain the term “bidentate ligand”. b) What is the coordination number of the [Fe(C2O4)3]3complex. c) Use your answer to part (b) to suggest what shape the [Fe(C2O4)3]3- complex is and draw it. ...
lect4b
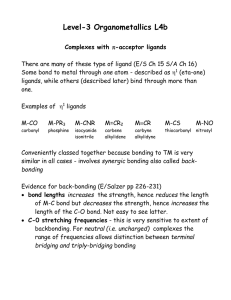
... Level-3 Organometallics L4b Complexes with -acceptor ligands There are many of these type of ligand (E/S Ch 15 S/A Ch 16) Some bond to metal through one atom - described as 1 (eta-one) ligands, while others (described later) bind through more than one. Examples of 1 ligands M-CO ...
... Level-3 Organometallics L4b Complexes with -acceptor ligands There are many of these type of ligand (E/S Ch 15 S/A Ch 16) Some bond to metal through one atom - described as 1 (eta-one) ligands, while others (described later) bind through more than one. Examples of 1 ligands M-CO ...
Soil solution part 3
... • Measure of deviation from standard T,P and ideal solutions • Activity (α) is a correction factor to account for non-ideality and is between 0 and 1 as solution concentration decreases, α 1 ...
... • Measure of deviation from standard T,P and ideal solutions • Activity (α) is a correction factor to account for non-ideality and is between 0 and 1 as solution concentration decreases, α 1 ...
in English
... mechanisms explaining the diversity of the reaction paths of alkylgallium complexes oxygenation were proposed. Moreover, the interesting results have been received for the oxygenation reactions of alkylaluminum derivatives with methyl ester of 2-pyrrolocarboxylic acid (metpyrrol-H) ligand. The final ...
... mechanisms explaining the diversity of the reaction paths of alkylgallium complexes oxygenation were proposed. Moreover, the interesting results have been received for the oxygenation reactions of alkylaluminum derivatives with methyl ester of 2-pyrrolocarboxylic acid (metpyrrol-H) ligand. The final ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... Part-A Answer all questions. Each question carries two marks: (10x2=20) 1. State Kramer’s theorem. 2. Orgel diagrams are constructed as a function of the Racah parameter B whereas Tanabe Suganodiagrams are constructed for a particular ratio of the Racah parameters B and C. Comment. 3. What is chelat ...
... Part-A Answer all questions. Each question carries two marks: (10x2=20) 1. State Kramer’s theorem. 2. Orgel diagrams are constructed as a function of the Racah parameter B whereas Tanabe Suganodiagrams are constructed for a particular ratio of the Racah parameters B and C. Comment. 3. What is chelat ...
Ru Complex Synthesis
... DNA is the target of anti-cancer metal drugs University of Richmond, November 2010 ...
... DNA is the target of anti-cancer metal drugs University of Richmond, November 2010 ...