C h e m g u i d e ... COMPLEX IONS - INTRODUCTION
... a) Use the diagram to explain what is meant by the term ligands. b) What is the essential feature of a molecule or ion which can serve as a ligand? c) What sort of bonding is there between the ligand and the metal ion? d) What is the coordination number of the aluminium in this ion? e) Explain what ...
... a) Use the diagram to explain what is meant by the term ligands. b) What is the essential feature of a molecule or ion which can serve as a ligand? c) What sort of bonding is there between the ligand and the metal ion? d) What is the coordination number of the aluminium in this ion? e) Explain what ...
Cu II complex - IONiC / VIPEr
... relationship that provides the selectivity for Cu(I). It also provides insight into potential mechanisms of heavy metal toxicity in this and other biochemical pathways. ...
... relationship that provides the selectivity for Cu(I). It also provides insight into potential mechanisms of heavy metal toxicity in this and other biochemical pathways. ...
Test No. 108: Complex Formation Ability in Water - Books
... The current should be measured at applied potentials in the range - 0.2 V to -1.0 V. In order to detect complexes which form slowly, it is necessary to allow the solutions to stand under a nitrogen atmosphere for a minimum period of 24 hours and, by re-examination of a sufficient number of samples, ...
... The current should be measured at applied potentials in the range - 0.2 V to -1.0 V. In order to detect complexes which form slowly, it is necessary to allow the solutions to stand under a nitrogen atmosphere for a minimum period of 24 hours and, by re-examination of a sufficient number of samples, ...
2 - A-Level Chemistry
... 20.0 cm3 of an acidified solution of H2O2 was found to react with exactly 15.7 cm3 of a 0.0180 mol dm–3 solution of potassium manganate(VII). Calculate the concentration, in g dm–3, of the solution of hydrogen peroxide. (If you have been unable to complete the overall equation in part (b)(i), assume ...
... 20.0 cm3 of an acidified solution of H2O2 was found to react with exactly 15.7 cm3 of a 0.0180 mol dm–3 solution of potassium manganate(VII). Calculate the concentration, in g dm–3, of the solution of hydrogen peroxide. (If you have been unable to complete the overall equation in part (b)(i), assume ...
Information regarding naming of Complex Ions Reference Sources: Naming Complex Ions
... For splitting of d orbitals due to ligands around central metal ion see: http://www.chemguide.co.uk/inorganic/complexions/colour2.html ...
... For splitting of d orbitals due to ligands around central metal ion see: http://www.chemguide.co.uk/inorganic/complexions/colour2.html ...
Complexometric Reactions (1)
... If the equilibrium is in the opposite direction, the constant is the reciprocal of the formation constant and is called the dissociation constant: ...
... If the equilibrium is in the opposite direction, the constant is the reciprocal of the formation constant and is called the dissociation constant: ...
Abstract Coordination Chemistry of Tetra(pyrazolyl)
... There is current interest in the coordination chemistry of simple AE4 pentadentate ligands that occupy one axial (A) and four equatorial (E) positions about a given transition metal center considering that systems capable of mediating spectacular organic transformations such as alkane oxidation have ...
... There is current interest in the coordination chemistry of simple AE4 pentadentate ligands that occupy one axial (A) and four equatorial (E) positions about a given transition metal center considering that systems capable of mediating spectacular organic transformations such as alkane oxidation have ...
Bioinorganic Chemistry
... comply with the following conditions: each compound has 1) empirical formula Pt(NH3)2Cl2, 2) is composed of discrete, monomeric ionic platinum(II) complex entities, and 3) contains only one type of cation and one type of anion. The answer must clearly reveal the composition of each discrete platinum ...
... comply with the following conditions: each compound has 1) empirical formula Pt(NH3)2Cl2, 2) is composed of discrete, monomeric ionic platinum(II) complex entities, and 3) contains only one type of cation and one type of anion. The answer must clearly reveal the composition of each discrete platinum ...
Jordan University of Science and Technology Abstract: Authors
... New eight lanthanide metal complexes were prepared. These complexes were characterized by elemental analysis, molar conductivity measurements, spectral analysis (1H NMR, FT-IR, UV?vis), luminescence and thermal gravimetric analysis. All Ln(III) complexes were 1:1 electrolytes as established by their ...
... New eight lanthanide metal complexes were prepared. These complexes were characterized by elemental analysis, molar conductivity measurements, spectral analysis (1H NMR, FT-IR, UV?vis), luminescence and thermal gravimetric analysis. All Ln(III) complexes were 1:1 electrolytes as established by their ...
Water as a Brønsted acid or base
... In practice, the character of the metal …. oxygen interaction varies with the nature of the metal ion. The configurations 7.6 and 7.7 have been established in the first hydration shell for dilute solutions of LiCl and NaCl by detailed neutron diffraction studies. In concentrated solutions, the plan ...
... In practice, the character of the metal …. oxygen interaction varies with the nature of the metal ion. The configurations 7.6 and 7.7 have been established in the first hydration shell for dilute solutions of LiCl and NaCl by detailed neutron diffraction studies. In concentrated solutions, the plan ...
Synthesis and Characterization of a Copper(I)
... with Ts from p-toluenesulfonic acid (TsCl). The resultant OTs is a better leaving group than OH to aid the synthesis of compound 2 in step 2. ...
... with Ts from p-toluenesulfonic acid (TsCl). The resultant OTs is a better leaving group than OH to aid the synthesis of compound 2 in step 2. ...
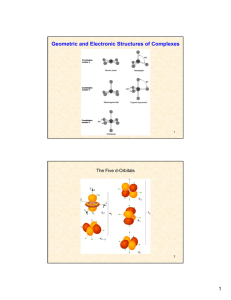
Geometric and Electronic Structures of Complexes
... the smaller the cation, the larger its hydration energy (which is closely related to the enthalpy of formation of [M(H2O)6]2+, for instance.) cations possessing CFSE show higher hydration energies than otherwise expected. CFSE therefore adds to the stabilization of the aqua complexes. V2+ and Ni2+ h ...
... the smaller the cation, the larger its hydration energy (which is closely related to the enthalpy of formation of [M(H2O)6]2+, for instance.) cations possessing CFSE show higher hydration energies than otherwise expected. CFSE therefore adds to the stabilization of the aqua complexes. V2+ and Ni2+ h ...
Available - Ggu.ac.in
... Metal-EDTA complex These data show that the standard enthalpy changes are indeed approximately equal for the two reactions and that the main reason why the chelate complex is so much more stable is that the standard entropy term is much less unfavourable, indeed, it is favourable in this instance. I ...
... Metal-EDTA complex These data show that the standard enthalpy changes are indeed approximately equal for the two reactions and that the main reason why the chelate complex is so much more stable is that the standard entropy term is much less unfavourable, indeed, it is favourable in this instance. I ...
© Ravi Divakaran THE CHELATE EFFECT Replacement of
... Note: Since ‘en’ is a bidentate ligand, each ‘en’ is equivalent to two NH3 ligands. In each of the above pairs, we see that the log value for complex with ‘en’ ligand is greater than that with NH3 ligands, meaning that they are more stable. The reason for the extra stability may be attributed to b ...
... Note: Since ‘en’ is a bidentate ligand, each ‘en’ is equivalent to two NH3 ligands. In each of the above pairs, we see that the log value for complex with ‘en’ ligand is greater than that with NH3 ligands, meaning that they are more stable. The reason for the extra stability may be attributed to b ...
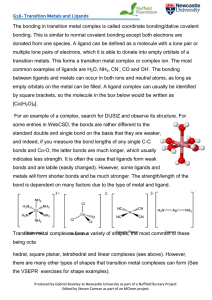
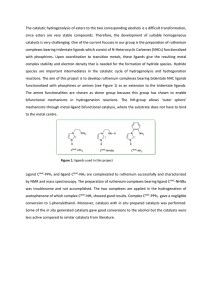
The catalytic hydrogenolysis of esters to the two corresponding
... with phosphines. Upon coordination to transition metals, these ligands give the resulting metal complex stability and electron density that is needed for the formation of hydride species. Hydride species are important intermediates in the catalytic cycle of hydrogenolysis and hydrogenation reactions ...
... with phosphines. Upon coordination to transition metals, these ligands give the resulting metal complex stability and electron density that is needed for the formation of hydride species. Hydride species are important intermediates in the catalytic cycle of hydrogenolysis and hydrogenation reactions ...
Critical Thinking Worksheet 9
... CHEM1612 Worksheet 9: Complexes Model 1: The Stability of Complexes Complexes contain a metal ion bonded to ligands. Most transition metal ions exist in aqueous solution as aqua complexes [M(OH2)m]n+ The stability of a complex can be measured using the stability constant or Kstab. This is just an eq ...
... CHEM1612 Worksheet 9: Complexes Model 1: The Stability of Complexes Complexes contain a metal ion bonded to ligands. Most transition metal ions exist in aqueous solution as aqua complexes [M(OH2)m]n+ The stability of a complex can be measured using the stability constant or Kstab. This is just an eq ...
03 Complexation equilibrium
... 1. Functional-analytical groups (FAG) - are specific groups which provide occurrence of donor-acceptor bond. ...
... 1. Functional-analytical groups (FAG) - are specific groups which provide occurrence of donor-acceptor bond. ...