
Organic Chemistry Chem 121: Topics
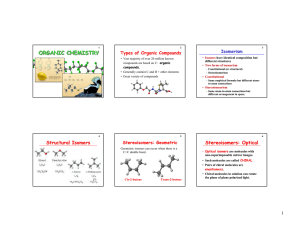
... which have the same molecular formula, but differ in the arrangement of their atoms, are called isomers. Constitutional (or structural) isomers differ in their bonding sequence. Stereoisomers differ only in the arrangement of the atoms in space. ...
... which have the same molecular formula, but differ in the arrangement of their atoms, are called isomers. Constitutional (or structural) isomers differ in their bonding sequence. Stereoisomers differ only in the arrangement of the atoms in space. ...
OrganicChemistryforAPlecture2010StudentVersioncompatibility
... Notice: There is no alkyne corresponding to the methane of the alkane series. That is b/c there must be at least 2 carbon atoms to form a triple bond. ...
... Notice: There is no alkyne corresponding to the methane of the alkane series. That is b/c there must be at least 2 carbon atoms to form a triple bond. ...
Organic Chemistry = the study of carbon and most carbon compounds
... Notice: There is no alkyne corresponding to the methane of the alkane series. That is b/c there must be at least 2 carbon atoms to form a triple bond. ...
... Notice: There is no alkyne corresponding to the methane of the alkane series. That is b/c there must be at least 2 carbon atoms to form a triple bond. ...
Unit 5 and 6 revsion - Deans Community High School
... (d) Draw the full structural formula for an isomer of buta-1,3-diene which contains only one double bond per molecule. ...
... (d) Draw the full structural formula for an isomer of buta-1,3-diene which contains only one double bond per molecule. ...
Organic Chemistry
... Alkynes, are hydrocarbons with carbon-carbon triple bonds. Alkynes two Sp hybridized orbitals and 2 p orbitals, and have bond angles of 180 degrees. unlike carbon-carbon single bonds, triple and double bonds do not have free bond rotation, and have shorter bond lengths. This is due to the rigidity o ...
... Alkynes, are hydrocarbons with carbon-carbon triple bonds. Alkynes two Sp hybridized orbitals and 2 p orbitals, and have bond angles of 180 degrees. unlike carbon-carbon single bonds, triple and double bonds do not have free bond rotation, and have shorter bond lengths. This is due to the rigidity o ...
CHEM 210 Nomenclature Lecture
... and a long chain. If the number of carbons in the ring is greater than or equal to the number of carbons in the longest chain, the compound is named as a cycloalkane. ...
... and a long chain. If the number of carbons in the ring is greater than or equal to the number of carbons in the longest chain, the compound is named as a cycloalkane. ...
Chapter 7 Carbon Chemistry Section 1 What is the atomic number of
... What % of known compounds contain carbon? ...
... What % of known compounds contain carbon? ...
Period 5
... • Has double or triple bonds with fewer hydrogen atoms for each carbon atom than a saturated hydrocarbon does. ...
... • Has double or triple bonds with fewer hydrogen atoms for each carbon atom than a saturated hydrocarbon does. ...
naming using more functional groups
... • with reference to the carbon that is directly bonded to a hydroxyl or a halogen – Primary = carbon atom is only bonded to one other carbon – Secondary = carbon atom is bonded to two other carbons – Tertiary = carbon atom is bonded to three other carbons ...
... • with reference to the carbon that is directly bonded to a hydroxyl or a halogen – Primary = carbon atom is only bonded to one other carbon – Secondary = carbon atom is bonded to two other carbons – Tertiary = carbon atom is bonded to three other carbons ...
Organic Chemistry
... Notice: There is no alkyne corresponding to the methane of the alkane series. That is b/c there must be at least 2 carbon atoms to form a triple bond. ...
... Notice: There is no alkyne corresponding to the methane of the alkane series. That is b/c there must be at least 2 carbon atoms to form a triple bond. ...
Organic Chemistry ppt 2012
... Notice: There is no alkyne corresponding to the methane of the alkane series. That is b/c there must be at least 2 carbon atoms to form a triple bond. ...
... Notice: There is no alkyne corresponding to the methane of the alkane series. That is b/c there must be at least 2 carbon atoms to form a triple bond. ...
ORGANIC CHEMISTRY
... generally occurs when a C atom has 4 different groups attached. Lactic acid isomers are nonsuperimposable ...
... generally occurs when a C atom has 4 different groups attached. Lactic acid isomers are nonsuperimposable ...
HYDROCARBONS HYDROCARBONS Types of Hydrocarbons
... 5. When both directions lead to the same lowest number for one of the substituents, the direction is chosen that gives the lowest possible number to one of the remaining substituents ...
... 5. When both directions lead to the same lowest number for one of the substituents, the direction is chosen that gives the lowest possible number to one of the remaining substituents ...
Organic Chemistry: Introduction
... • with reference to the carbon that is directly bonded to an alcohol group or a halogen: – Primary = carbon atom is only bonded to one other carbon – Secondary = carbon atom is bonded to two other carbons – Tertiary = carbon atom is bonded to three other carbons ...
... • with reference to the carbon that is directly bonded to an alcohol group or a halogen: – Primary = carbon atom is only bonded to one other carbon – Secondary = carbon atom is bonded to two other carbons – Tertiary = carbon atom is bonded to three other carbons ...
unit (7) organic compounds: hydrocarbons
... The first four classes of organic compounds in Table 7.1 are known as hydrocarbons. A hydrocarbon is a compound composed of entirely carbon and hydrogen atoms. Hydrocarbons are classified as aromatic compounds (containing benzene rings) and aliphatics (all other hydrocarbons). ...
... The first four classes of organic compounds in Table 7.1 are known as hydrocarbons. A hydrocarbon is a compound composed of entirely carbon and hydrogen atoms. Hydrocarbons are classified as aromatic compounds (containing benzene rings) and aliphatics (all other hydrocarbons). ...
CHEM 210 Nomenclature Lecture 1
... • If two or more identical substituents are bonded to the longest chain, use prefixes to indicate how many: di- for two groups, trifor three groups, tetra- for four groups, and so forth. ...
... • If two or more identical substituents are bonded to the longest chain, use prefixes to indicate how many: di- for two groups, trifor three groups, tetra- for four groups, and so forth. ...
organic chemistry
... • (A)A straight-chain alkane is identified by the prefix n- for "normal" in the common naming system. (B) A branched-chain alkane isomer is identified by the prefix iso- for "isomer" in the common naming system. In the IUPAC name, isobutane is 2-methylpropane. (Carbon bonds are actually the same len ...
... • (A)A straight-chain alkane is identified by the prefix n- for "normal" in the common naming system. (B) A branched-chain alkane isomer is identified by the prefix iso- for "isomer" in the common naming system. In the IUPAC name, isobutane is 2-methylpropane. (Carbon bonds are actually the same len ...
Organic Compounds
... The formula for alkanes is CnH2n+2. The names are derived by taking the prefix for the number of carbons it contains and adding the suffix – ane. Ex: 4 carbon alkane = But-ane or butane. ...
... The formula for alkanes is CnH2n+2. The names are derived by taking the prefix for the number of carbons it contains and adding the suffix – ane. Ex: 4 carbon alkane = But-ane or butane. ...
Part 1
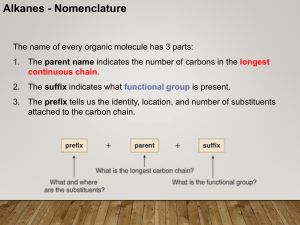
... Rule #2: Number the carbons in the main chain Number chain to minimize the position/number of the following in order of priority: a) thing you’re naming the compound after (double bond if alkene; -OH group if alcohol, etc) note: for multiple double bonds -diene, -triene, -tetraene ...
... Rule #2: Number the carbons in the main chain Number chain to minimize the position/number of the following in order of priority: a) thing you’re naming the compound after (double bond if alkene; -OH group if alcohol, etc) note: for multiple double bonds -diene, -triene, -tetraene ...
C1_Revision_Sheets[1] - Chew Valley School | Intranet Homepage
... C1 REVISION – CHAPTER 1 – FUNDAMENTAL IDEAS Draw the symbol for sodium include its atomic mass and atomic number (what do they tell us) ...
... C1 REVISION – CHAPTER 1 – FUNDAMENTAL IDEAS Draw the symbol for sodium include its atomic mass and atomic number (what do they tell us) ...
chapter03 - FacStaff Home Page for CBU
... Glycerol is a 3-carbon alcohol. Fatty acids are long unbranched hydrocarbon chain with a carboxyl group (COOH) at one end. Triglyceride (triacylglycerol) is a synonym for fat. Saturated fats have a maximum number of hydrogen atoms in the chain, and are usually are solid at room temperature. Unsatura ...
... Glycerol is a 3-carbon alcohol. Fatty acids are long unbranched hydrocarbon chain with a carboxyl group (COOH) at one end. Triglyceride (triacylglycerol) is a synonym for fat. Saturated fats have a maximum number of hydrogen atoms in the chain, and are usually are solid at room temperature. Unsatura ...
Alkane
In organic chemistry, an alkane, or paraffin (a historical name that also has other meanings), is a saturated hydrocarbon. Alkanes consist only of hydrogen and carbon atoms and all bonds are single bonds. Alkanes (technically, always acyclic or open-chain compounds) have the general chemical formula CnH2n+2. For example, Methane is CH4, in which n=1 (n being the number of Carbon atoms). Alkanes belong to a homologous series of organic compounds in which the members differ by a molecular mass of 14.03u (mass of a methanediyl group, —CH2—, one carbon atom of mass 12.01u, and two hydrogen atoms of mass ≈1.01u each). There are two main commercial sources: petroleum (crude oil) and natural gas.Each carbon atom has 4 bonds (either C-H or C-C bonds), and each hydrogen atom is joined to a carbon atom (H-C bonds). A series of linked carbon atoms is known as the carbon skeleton or carbon backbone. The number of carbon atoms is used to define the size of the alkane e.g., C2-alkane.An alkyl group, generally abbreviated with the symbol R, is a functional group or side-chain that, like an alkane, consists solely of single-bonded carbon and hydrogen atoms, for example a methyl or ethyl group.The simplest possible alkane (the parent molecule) is methane, CH4. There is no limit to the number of carbon atoms that can be linked together, the only limitation being that the molecule is acyclic, is saturated, and is a hydrocarbon. Waxes include examples of larger alkanes where the number of carbons in the carbon backbone is greater than about 17, above which the compounds are solids at standard ambient temperature and pressure (SATP).Alkanes are not very reactive and have little biological activity. All alkanes are colourless and odourless. Alkanes can be viewed as a molecular tree upon which can be hung the more biologically active/reactive portions (functional groups) of the molecule.



















![C1_Revision_Sheets[1] - Chew Valley School | Intranet Homepage](http://s1.studyres.com/store/data/003668408_1-6e6cbb7760f896a3d0f766960e7af724-300x300.png)
