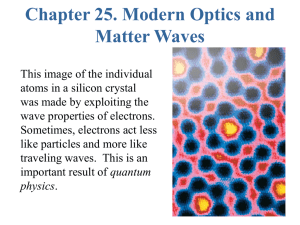
Chapt25_VGO
... • Hydrogen is the simplest atom, with one electron orbiting a proton, and it also has the simplest atomic spectrum. • The emission lines have wavelengths which correspond to two integers, m and n. • Every line in the hydrogen spectrum has a wavelength given by ...
... • Hydrogen is the simplest atom, with one electron orbiting a proton, and it also has the simplest atomic spectrum. • The emission lines have wavelengths which correspond to two integers, m and n. • Every line in the hydrogen spectrum has a wavelength given by ...
Chem 30A Fa_06 FE Review
... Name the following compounds using IUPAC systematic nomenclature. (a) SO3 : ___________________________________ (b) FeSO4: __________________________________ (c) HC2H3O2: _________________________________ (d) Ca3(PO4)2: __________________________________ (e) Hg2Cl2: _________________________________ ...
... Name the following compounds using IUPAC systematic nomenclature. (a) SO3 : ___________________________________ (b) FeSO4: __________________________________ (c) HC2H3O2: _________________________________ (d) Ca3(PO4)2: __________________________________ (e) Hg2Cl2: _________________________________ ...
lecture notes, pages 4-5
... Microscopic particles, like electrons, whose �’s are on the order of their environment do not obey classical equations of motion. Electrons must be treated like waves to describe their behavior. 1927 Erwin Schrödinger wrote an equation of motion for particles (like electrons) that account for their ...
... Microscopic particles, like electrons, whose �’s are on the order of their environment do not obey classical equations of motion. Electrons must be treated like waves to describe their behavior. 1927 Erwin Schrödinger wrote an equation of motion for particles (like electrons) that account for their ...
Honors Chemistry
... Light as Energy: Planck derived a formula that expresses the energy of a photon at any given frequency Formula 1: ...
... Light as Energy: Planck derived a formula that expresses the energy of a photon at any given frequency Formula 1: ...
DARLLENWCH Y DARN ISOD AC ATEBWCH Y CWESTIYNAU SY
... however, that the quantum constant formulated by the German physicist Max Planck has dimensions which, when combined with the mass and charge of the electron, produce a measure of length. Numerically, the measure is close to the known size of atoms. This encouraged Bohr to use Planck's constant in s ...
... however, that the quantum constant formulated by the German physicist Max Planck has dimensions which, when combined with the mass and charge of the electron, produce a measure of length. Numerically, the measure is close to the known size of atoms. This encouraged Bohr to use Planck's constant in s ...
Chapter 12 Worksheet
... 3. According to Heisenberg uncertainty principle; a. the momentum of a particle cannot be measured precisely b. neither the position nor the momentum can be measured precisely c. the position and the momentum of a particle can be measured precisely, but not at the same time d. the positon of a parti ...
... 3. According to Heisenberg uncertainty principle; a. the momentum of a particle cannot be measured precisely b. neither the position nor the momentum can be measured precisely c. the position and the momentum of a particle can be measured precisely, but not at the same time d. the positon of a parti ...
5. Lectures on Spectroscopy and Atomic Physics.
... The continuous spectrum is produced in part by electrons that are not necessarily confined to bound orbits, but are relatively free to wander around. Also, in the case in a solid or gas under high pressure, atoms and molecules bound together by electric fields and shared electrons store energy in th ...
... The continuous spectrum is produced in part by electrons that are not necessarily confined to bound orbits, but are relatively free to wander around. Also, in the case in a solid or gas under high pressure, atoms and molecules bound together by electric fields and shared electrons store energy in th ...
Document
... Consider the radiation as a gas of photon. Photons move randomly like molecules in a gas and have wide range of energies but same velocity Statistics of photons is described by Bose-Einstein. We can talk about intensity and temperature in the same way as density and temperature. ...
... Consider the radiation as a gas of photon. Photons move randomly like molecules in a gas and have wide range of energies but same velocity Statistics of photons is described by Bose-Einstein. We can talk about intensity and temperature in the same way as density and temperature. ...
AP Chemistry
... What is the energy in joules of one photon of microwave radiation with a wavelength 0..124m? ...
... What is the energy in joules of one photon of microwave radiation with a wavelength 0..124m? ...
constructive - Purdue Physics
... protons is called Z, the atomic number. The total number of protons and neutrons is called A, the mass number. The number of neutrons is therefore A-Z. The neutral atom has Z electrons which determine the chemical properties of the element. Therefore Z determines which type of element the atom is (H ...
... protons is called Z, the atomic number. The total number of protons and neutrons is called A, the mass number. The number of neutrons is therefore A-Z. The neutral atom has Z electrons which determine the chemical properties of the element. Therefore Z determines which type of element the atom is (H ...
Notes - Photons, the Photoelectric Effect and the Compton Effect (ppt)
... • Experimental data shows there is a minimum (cutoff frequency) that the light must have. • Classical physics predicts that the kinetic energy of the ejected electrons should increase with the intensity of the light. • Again, experimental data shows this is not the case; increasing the intensity of ...
... • Experimental data shows there is a minimum (cutoff frequency) that the light must have. • Classical physics predicts that the kinetic energy of the ejected electrons should increase with the intensity of the light. • Again, experimental data shows this is not the case; increasing the intensity of ...
photoelectric effect
... known as the photoelectric effect. Einstein’s explanation of this effect in 1905 (the year he also developed Special Relativity!) is one of the cornerstones of quantum physics. According to the classical theory of electromagnetic fields, the intensity of a light wave is directly proportional to the ...
... known as the photoelectric effect. Einstein’s explanation of this effect in 1905 (the year he also developed Special Relativity!) is one of the cornerstones of quantum physics. According to the classical theory of electromagnetic fields, the intensity of a light wave is directly proportional to the ...
chapter27
... They followed this with extensive diffraction measurements from various materials The wavelength of the electrons calculated from the diffraction data agreed with the expected de Broglie wavelength This confirmed the wave nature of electrons Other experimenters have confirmed the wave nature of othe ...
... They followed this with extensive diffraction measurements from various materials The wavelength of the electrons calculated from the diffraction data agreed with the expected de Broglie wavelength This confirmed the wave nature of electrons Other experimenters have confirmed the wave nature of othe ...
Chapter 27 - Planet Holloway
... nickel target They followed this with extensive diffraction measurements from various materials The wavelength of the electrons calculated from the diffraction data agreed with the expected de Broglie wavelength This confirmed the wave nature of electrons Other experimenters have confirmed the wave ...
... nickel target They followed this with extensive diffraction measurements from various materials The wavelength of the electrons calculated from the diffraction data agreed with the expected de Broglie wavelength This confirmed the wave nature of electrons Other experimenters have confirmed the wave ...
Chapter 5 Practice Section 5-1 Discuss the placement (if any) of
... What is the wavelength of radiation with a frequency of 2.3 x 1014 Hz? What is the frequency of radiation with a wavelength of 1.8 x 10-9 m? Rank in order of increasing energy: Purple light, x-rays, Microwaves Rank the above in order of increasing frequency. Rank the above in order of increasing wav ...
... What is the wavelength of radiation with a frequency of 2.3 x 1014 Hz? What is the frequency of radiation with a wavelength of 1.8 x 10-9 m? Rank in order of increasing energy: Purple light, x-rays, Microwaves Rank the above in order of increasing frequency. Rank the above in order of increasing wav ...
Energy and Matter - Hicksville Public Schools
... All waves can be described by their wavelength (), frequency (), amplitude, and speed. The wavelength and the frequency are indirectly related to each other – when the wavelength is short, the frequency is high and vise versa. ...
... All waves can be described by their wavelength (), frequency (), amplitude, and speed. The wavelength and the frequency are indirectly related to each other – when the wavelength is short, the frequency is high and vise versa. ...
particlephysics
... The angle of deflection is determined by the K.E. of the alpha particle. The “no – go “ zone behind the nucleus gets smaller the larger the K.E. of the alpha particles ...
... The angle of deflection is determined by the K.E. of the alpha particle. The “no – go “ zone behind the nucleus gets smaller the larger the K.E. of the alpha particles ...
Slide 1
... photons and was a phenomenal success. • Yukawa generalized QED to the nuclear force and predicted a mass about the same as the mass from cosmic rays – but it interacted stongly with the nucleus. • A non-interacting particle should not be made in nuclear collisions. • The pion was discovered a bit la ...
... photons and was a phenomenal success. • Yukawa generalized QED to the nuclear force and predicted a mass about the same as the mass from cosmic rays – but it interacted stongly with the nucleus. • A non-interacting particle should not be made in nuclear collisions. • The pion was discovered a bit la ...
Bremsstrahlung
Bremsstrahlung (German pronunciation: [ˈbʁɛmsˌʃtʁaːlʊŋ], from bremsen ""to brake"" and Strahlung ""radiation"", i.e. ""braking radiation"" or ""deceleration radiation"") is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into a photon, thus satisfying the law of conservation of energy. The term is also used to refer to the process of producing the radiation. Bremsstrahlung has a continuous spectrum, which becomes more intense and whose peak intensity shifts toward higher frequencies as the change of the energy of the accelerated particles increases.Strictly speaking, braking radiation is any radiation due to the acceleration of a charged particle, which includes synchrotron radiation, cyclotron radiation, and the emission of electrons and positrons during beta decay. However, the term is frequently used in the more narrow sense of radiation from electrons (from whatever source) slowing in matter.Bremsstrahlung emitted from plasma is sometimes referred to as free/free radiation. This refers to the fact that the radiation in this case is created by charged particles that are free both before and after the deflection (acceleration) that caused the emission.