![3 — Blackbody Radiation [Revision : 1.5]](http://s1.studyres.com/store/data/005908504_1-5005bdffc2e5f9c6e0687c31f49c7e9d-300x300.png)
3 — Blackbody Radiation [Revision : 1.5]
... – In the UV, visible and IR parts of spectrum of many stars, Fλ is crudely approximated as a blackbody • Blackbody radiation – Blackbody (BB) is an object that absorbs all radiation falling on it – In thermal equilibrium at temperature T , radiation re-emitted by BB has a unique Fλ that depends only ...
... – In the UV, visible and IR parts of spectrum of many stars, Fλ is crudely approximated as a blackbody • Blackbody radiation – Blackbody (BB) is an object that absorbs all radiation falling on it – In thermal equilibrium at temperature T , radiation re-emitted by BB has a unique Fλ that depends only ...
HW 2-1 Review Chap 2 Key
... Use the helium-4 isotope to define atomic number and mass number. Why does a knowledge of atomic number enable us to deduce the number of electrons present in an atom? Helium–4, written 42 He , has atomic number 2 because it has 2 protons (number at lower left) and mass number 4 because it has a tot ...
... Use the helium-4 isotope to define atomic number and mass number. Why does a knowledge of atomic number enable us to deduce the number of electrons present in an atom? Helium–4, written 42 He , has atomic number 2 because it has 2 protons (number at lower left) and mass number 4 because it has a tot ...
264-lecture-2015-10
... Quantum Mechanics Mnemonic for p “Now I need a drink, alcoholic of course, after the heavy lectures involving quantum mechanics.” ...
... Quantum Mechanics Mnemonic for p “Now I need a drink, alcoholic of course, after the heavy lectures involving quantum mechanics.” ...
FINAL REVIEW 1st SEMESTER 2014-2015
... aluminum acetate + sodium hydroxide → aluminum hydroxide + sodium acetate ________________________________________________________ ...
... aluminum acetate + sodium hydroxide → aluminum hydroxide + sodium acetate ________________________________________________________ ...
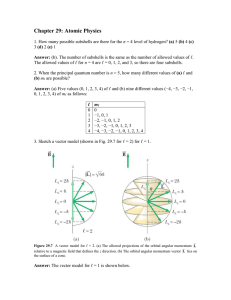
Chapter 29: Atomic Physics
... change and (ii), the minimum wavelength of the bremsstrahlung (a) increases, (b) decreases, or (c) does not change. Answer: (i), (c). The wavelengths of the characteristic x-rays are determined by the separation between energy levels in the atoms of the target, which is unrelated to the energy with ...
... change and (ii), the minimum wavelength of the bremsstrahlung (a) increases, (b) decreases, or (c) does not change. Answer: (i), (c). The wavelengths of the characteristic x-rays are determined by the separation between energy levels in the atoms of the target, which is unrelated to the energy with ...
Unit 2 Intro Worksheet - Coral Gables Senior High
... 2. states the impossibility of knowing both velocity and position of a moving particle at the same time ...
... 2. states the impossibility of knowing both velocity and position of a moving particle at the same time ...
OBJECTIVE WORKSHEET Quantum Theory 1. How did
... 2. What does it mean when a scientist says, "the energies of electrons are quantized." 3. How many energy levels for electrons does the chapter discuss? 4. Who discovered the QUANTUM MECHANICAL MODEL? 5. What shape does the s and p orbitals have? 6. What does "n" stand for when we discuss atomic orb ...
... 2. What does it mean when a scientist says, "the energies of electrons are quantized." 3. How many energy levels for electrons does the chapter discuss? 4. Who discovered the QUANTUM MECHANICAL MODEL? 5. What shape does the s and p orbitals have? 6. What does "n" stand for when we discuss atomic orb ...
Ch 6 Outline
... Limitations of the Bohr model Calculate E, , of the radiation….determine whether it is emitted or absorbed from n=5 to n=3 from n=3 to n=6 from n=6 to n=4 ...
... Limitations of the Bohr model Calculate E, , of the radiation….determine whether it is emitted or absorbed from n=5 to n=3 from n=3 to n=6 from n=6 to n=4 ...
L-J Chemistry 1 Quiz 25 1 A property that depends on the amount of
... the amount of matter Research carried out to solve a specific problem A cube that is 10 dm on each side =(equals 1 liter) Atomic mass of a substance in grams Device to break light down into a spectrum Electrons in the outer level of an atom that can be gained, shared or lost Energy added to an atom ...
... the amount of matter Research carried out to solve a specific problem A cube that is 10 dm on each side =(equals 1 liter) Atomic mass of a substance in grams Device to break light down into a spectrum Electrons in the outer level of an atom that can be gained, shared or lost Energy added to an atom ...
Modern physics 2330
... 11- ( ) Davisson-Germer experiment (1927) is a direct experimental proof that the electron charge is, e=1.6x10-19C. 12- ( ).The electron of the Bohr atom forms a standing wave around the nucleus. 13- ( ) The Heisenberg uncertainty principle states that, position and conjugate momentum can not be mea ...
... 11- ( ) Davisson-Germer experiment (1927) is a direct experimental proof that the electron charge is, e=1.6x10-19C. 12- ( ).The electron of the Bohr atom forms a standing wave around the nucleus. 13- ( ) The Heisenberg uncertainty principle states that, position and conjugate momentum can not be mea ...
Bremsstrahlung
Bremsstrahlung (German pronunciation: [ˈbʁɛmsˌʃtʁaːlʊŋ], from bremsen ""to brake"" and Strahlung ""radiation"", i.e. ""braking radiation"" or ""deceleration radiation"") is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into a photon, thus satisfying the law of conservation of energy. The term is also used to refer to the process of producing the radiation. Bremsstrahlung has a continuous spectrum, which becomes more intense and whose peak intensity shifts toward higher frequencies as the change of the energy of the accelerated particles increases.Strictly speaking, braking radiation is any radiation due to the acceleration of a charged particle, which includes synchrotron radiation, cyclotron radiation, and the emission of electrons and positrons during beta decay. However, the term is frequently used in the more narrow sense of radiation from electrons (from whatever source) slowing in matter.Bremsstrahlung emitted from plasma is sometimes referred to as free/free radiation. This refers to the fact that the radiation in this case is created by charged particles that are free both before and after the deflection (acceleration) that caused the emission.