The Larmor Formula
... Bremsstrahlung (~Braking radiation) come from the acceleration associated with electrostatic collisions between charged particles (called Coulomb collisions) Note that the electrostatic force is long range E~1/r2 – thus electrostatic collisions between charged particles is a smooth continuous proces ...
... Bremsstrahlung (~Braking radiation) come from the acceleration associated with electrostatic collisions between charged particles (called Coulomb collisions) Note that the electrostatic force is long range E~1/r2 – thus electrostatic collisions between charged particles is a smooth continuous proces ...
Chapter 4 Arrangement of Electrons in Atoms
... • Studied the absorption of light by ___________ • Absorption of light at definite wavelengths corresponds to the definite changes in the E of the _______________ • Electrons can circle the nucleus at ___________ _____________ distances…only in allowed paths, or orbits (Satellite model) • Energy of ...
... • Studied the absorption of light by ___________ • Absorption of light at definite wavelengths corresponds to the definite changes in the E of the _______________ • Electrons can circle the nucleus at ___________ _____________ distances…only in allowed paths, or orbits (Satellite model) • Energy of ...
Exercise Sheet 1 to Particle Physics I
... 1) Use the Particle Data Group (PDG) webpage (or other sources of information) to express the following quantities in the elementary particle physics natural units (i.e. in proper eV units using h̄ = c = 1): atomic radius (1 Å), nucleon radius (1 fm = typical size of atomic nuclei) classic electron ...
... 1) Use the Particle Data Group (PDG) webpage (or other sources of information) to express the following quantities in the elementary particle physics natural units (i.e. in proper eV units using h̄ = c = 1): atomic radius (1 Å), nucleon radius (1 fm = typical size of atomic nuclei) classic electron ...
SNC 1D0 – Chemistry Take Home Quiz
... 2. Identify each of the following as a physical change (P) or chemical change (C) and give a reason. Observed event P or C Reason (ex. Change of state, change in colour . .) Shredding paper Toasting marshmallows Cooking an egg Popsicle melting 3. Draw the classification of matter chart in detail wit ...
... 2. Identify each of the following as a physical change (P) or chemical change (C) and give a reason. Observed event P or C Reason (ex. Change of state, change in colour . .) Shredding paper Toasting marshmallows Cooking an egg Popsicle melting 3. Draw the classification of matter chart in detail wit ...
electromagnetic spectrum and flame tests
... levels to the second energy level. Ultraviolet radiation is emitted when the electron moves from the second through sixth energy levels to the first energy level. Infrared radiation is emitted when the electron moves from the fourth through sixth energy levels to the third energy level. Complete the ...
... levels to the second energy level. Ultraviolet radiation is emitted when the electron moves from the second through sixth energy levels to the first energy level. Infrared radiation is emitted when the electron moves from the fourth through sixth energy levels to the third energy level. Complete the ...
Spectra and atomic structure
... If we look at the spectrum of atomic hydrogen we can see that it is made up of series of lines. This arrangement of lines is unique to hydrogen, other monatomic gases have a line spectrum but no other element shows the same spectrum as hydrogen and it is sensible to suppose that the spectrum somehow ...
... If we look at the spectrum of atomic hydrogen we can see that it is made up of series of lines. This arrangement of lines is unique to hydrogen, other monatomic gases have a line spectrum but no other element shows the same spectrum as hydrogen and it is sensible to suppose that the spectrum somehow ...
... I 10. (1 0) The decay between two excited states of the nucle~isof 4 ' ~ iemits gamma ray of 1.3117 MeV. Tht luppe, state has a lifetime of 1.4ps, the lower state 3.0 ps. A) What is the fractional uncertainty AEIE in tht energy of the gainma ray? B) What is the percentage spread in wavelength of the ...
A system consist of two particles,each of which has two possible
... with energies Eo and 2Eo .Write the complete expression for the partition function if: (a) The particle are distinguishable. (b) The particle obey Maxwell-Boltzmann statistics. (c) The particle obey Fermi-Dirac statistics. (d) The particle obey Bose-Einstein statistics. 2.When a closed cubic box of ...
... with energies Eo and 2Eo .Write the complete expression for the partition function if: (a) The particle are distinguishable. (b) The particle obey Maxwell-Boltzmann statistics. (c) The particle obey Fermi-Dirac statistics. (d) The particle obey Bose-Einstein statistics. 2.When a closed cubic box of ...
27-3 A Photoelectric Effect Example
... from reaching the second plate (this is known as the stopping potential). In this case, the stopping potential is 1.40 V, so the maximum kinetic energy of the electrons is 1.40 eV. (c) Now that we know the photon energy and the maximum kinetic energy of the electrons, we can use Equation 27.3 to fin ...
... from reaching the second plate (this is known as the stopping potential). In this case, the stopping potential is 1.40 V, so the maximum kinetic energy of the electrons is 1.40 eV. (c) Now that we know the photon energy and the maximum kinetic energy of the electrons, we can use Equation 27.3 to fin ...
How do chemists determine the formula of
... • Do you know the mass of iron used? (g Fe) • Do you know the mass of oxygen used? (g O) • Could you convert both grams to moles? • Could you find the molar ratio of Fe to O? What would that tell you about the formula? ...
... • Do you know the mass of iron used? (g Fe) • Do you know the mass of oxygen used? (g O) • Could you convert both grams to moles? • Could you find the molar ratio of Fe to O? What would that tell you about the formula? ...
WODSS SCIENCE SCH 3UI Empirical and Molecular Formula
... John Dalton stated that _____________________________________________________________ ________________________________________________________________________________ Water has the chemical formula H2O. In terms of mass, its molecule is always made up of ________ hydrogen and __________ of oxygen. E ...
... John Dalton stated that _____________________________________________________________ ________________________________________________________________________________ Water has the chemical formula H2O. In terms of mass, its molecule is always made up of ________ hydrogen and __________ of oxygen. E ...
1 The Photoelectric Effect 2 Line Spectra and Energy Levels
... Bohr model : a mechanical model of the hydrogen atom proposed by Bohr using the relationship between spectral wavelengths and energy levels. stable orbits: path of the electron revolving around the nucleus that do not decay and do not emit radiation (contrary to classical electromagnetic theory). qu ...
... Bohr model : a mechanical model of the hydrogen atom proposed by Bohr using the relationship between spectral wavelengths and energy levels. stable orbits: path of the electron revolving around the nucleus that do not decay and do not emit radiation (contrary to classical electromagnetic theory). qu ...
Chapter 28 notes
... Bohr model : a mechanical model of the hydrogen atom proposed by Bohr using the relationship between spectral wavelengths and energy levels. stable orbits: path of the electron revolving around the nucleus that do not decay and do not emit radiation (contrary to classical electromagnetic theory). qu ...
... Bohr model : a mechanical model of the hydrogen atom proposed by Bohr using the relationship between spectral wavelengths and energy levels. stable orbits: path of the electron revolving around the nucleus that do not decay and do not emit radiation (contrary to classical electromagnetic theory). qu ...
Physics 2DL Lectures
... (J) Franck & (G) Hertz Experiment Current decreases because many Electrons lose energy due to inelastic Scattering with the Hg atom in tube And therefore can not overcome the ...
... (J) Franck & (G) Hertz Experiment Current decreases because many Electrons lose energy due to inelastic Scattering with the Hg atom in tube And therefore can not overcome the ...
Physics 120 Homework Set #1 (due Sunday
... b) Does the principle describe a property of a quantum object (e.g. electron) or the limitations of an action (i.e. observation or measurement)? Explain. The limit posed by the Uncertainty Principle is not a consequence of some deficiency in the experimental techniques. Rather, it signifies that an ...
... b) Does the principle describe a property of a quantum object (e.g. electron) or the limitations of an action (i.e. observation or measurement)? Explain. The limit posed by the Uncertainty Principle is not a consequence of some deficiency in the experimental techniques. Rather, it signifies that an ...
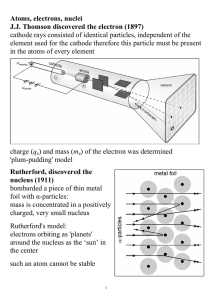
02 Atomic Structure
... (c) the full nuclear charge of the target atom is partially screened by its electron (d) all of the above Q 10. The conclusions of Rutherford scattering experiment does not include: (a) -particle can come within a distance of the order of 10-14 m of the nucleus (b) The radius of the nucleus is less ...
... (c) the full nuclear charge of the target atom is partially screened by its electron (d) all of the above Q 10. The conclusions of Rutherford scattering experiment does not include: (a) -particle can come within a distance of the order of 10-14 m of the nucleus (b) The radius of the nucleus is less ...
Chemistry Name______________________________________
... why objects only emit certain frequencies of light at a given temp a small specific amount of energy. (min energy gained or lost by an atom) building a wall with bricks. Gets bigger or smaller in the increment the size of the brick bundle of light energy.(a quantum of energy in ...
... why objects only emit certain frequencies of light at a given temp a small specific amount of energy. (min energy gained or lost by an atom) building a wall with bricks. Gets bigger or smaller in the increment the size of the brick bundle of light energy.(a quantum of energy in ...
Bremsstrahlung
Bremsstrahlung (German pronunciation: [ˈbʁɛmsˌʃtʁaːlʊŋ], from bremsen ""to brake"" and Strahlung ""radiation"", i.e. ""braking radiation"" or ""deceleration radiation"") is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into a photon, thus satisfying the law of conservation of energy. The term is also used to refer to the process of producing the radiation. Bremsstrahlung has a continuous spectrum, which becomes more intense and whose peak intensity shifts toward higher frequencies as the change of the energy of the accelerated particles increases.Strictly speaking, braking radiation is any radiation due to the acceleration of a charged particle, which includes synchrotron radiation, cyclotron radiation, and the emission of electrons and positrons during beta decay. However, the term is frequently used in the more narrow sense of radiation from electrons (from whatever source) slowing in matter.Bremsstrahlung emitted from plasma is sometimes referred to as free/free radiation. This refers to the fact that the radiation in this case is created by charged particles that are free both before and after the deflection (acceleration) that caused the emission.