Chapter 13
... concentration of a reactant is increased. In some reactions, however, the rate is unaffected by the concentration of a particular reactant, as long as it is present at some concentration. ...
... concentration of a reactant is increased. In some reactions, however, the rate is unaffected by the concentration of a particular reactant, as long as it is present at some concentration. ...
Formic acid oxidation reaction on a PdxNiy bimetallic nanoparticle
... and graphene ribbon itself served as the reducing reagent, stabilizer, and catalyst support for Pd. And the as-synthesized Pd/GR electrocatalysts showed increased electrochemical surface area and significantly enhanced catalytic activity for FAOR compared with the conventional Pd/C electrocatalysts ...
... and graphene ribbon itself served as the reducing reagent, stabilizer, and catalyst support for Pd. And the as-synthesized Pd/GR electrocatalysts showed increased electrochemical surface area and significantly enhanced catalytic activity for FAOR compared with the conventional Pd/C electrocatalysts ...
9701/04 - StudyGuide.PK
... Permission to reproduce items where third-party owned material protected by copyright is included has been sought and cleared where possible. Every reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included ...
... Permission to reproduce items where third-party owned material protected by copyright is included has been sought and cleared where possible. Every reasonable effort has been made by the publisher (UCLES) to trace copyright holders, but if any items requiring clearance have unwittingly been included ...
ap chemistry 2005/2006
... 3-4 days of lecture focused on the key objectives listed in the syllabus, including teacher demonstrations 1-2 days of lab activity. Labs may exceed one 90 minute class, depending on the requirements of the specific lab activity. In addition, some sections/objectives are more conducive to lab ac ...
... 3-4 days of lecture focused on the key objectives listed in the syllabus, including teacher demonstrations 1-2 days of lab activity. Labs may exceed one 90 minute class, depending on the requirements of the specific lab activity. In addition, some sections/objectives are more conducive to lab ac ...
Chapter 4 Student Notes
... o As HCl is added, the magnesium hydroxide dissolves, and a clear solution containing Mg 2+ and Cl1- ions is formed. o Molecular equation: Mg(OH)2 (s) + 2 HCl(aq) MgCl2 (aq) + 2 H2O(l) o Complete ionic equation: Mg(OH)2 (s) + 2 H1+(aq) + Cl1-(aq) Mg2+(aq) + 2 Cl1-(aq) + 2 H2O(l) o Net ionic ...
... o As HCl is added, the magnesium hydroxide dissolves, and a clear solution containing Mg 2+ and Cl1- ions is formed. o Molecular equation: Mg(OH)2 (s) + 2 HCl(aq) MgCl2 (aq) + 2 H2O(l) o Complete ionic equation: Mg(OH)2 (s) + 2 H1+(aq) + Cl1-(aq) Mg2+(aq) + 2 Cl1-(aq) + 2 H2O(l) o Net ionic ...
File
... The reaction of methane and water is one way to prepare hydrogen. CH4(g) + 2 H2O(g) CO2(g) + 4 H2(g) If 0.320 mol of methane reacts with 0.530 mol of water, what is the limiting reagent? a. CH4(g) c. H2(g) b. CO2(g) d. H2O(g) ...
... The reaction of methane and water is one way to prepare hydrogen. CH4(g) + 2 H2O(g) CO2(g) + 4 H2(g) If 0.320 mol of methane reacts with 0.530 mol of water, what is the limiting reagent? a. CH4(g) c. H2(g) b. CO2(g) d. H2O(g) ...
Gas-phase study of the reactivity of optical coating desktop-size extreme-ultraviolet laser
... equipment is given below. MmOn (M = Si, Ti, Hf, or Zr) clusters are generated by laser ablation with a focused 532 nm laser (Nd3+ : YAG, 10 Hz, 5 – 8 mJ/ cm2, 8 ns duration) onto a 12 mm diameter spring-loaded metal disk in the presence of a pulsed helium carrier gas mixed with 5% O2, controlled by ...
... equipment is given below. MmOn (M = Si, Ti, Hf, or Zr) clusters are generated by laser ablation with a focused 532 nm laser (Nd3+ : YAG, 10 Hz, 5 – 8 mJ/ cm2, 8 ns duration) onto a 12 mm diameter spring-loaded metal disk in the presence of a pulsed helium carrier gas mixed with 5% O2, controlled by ...
Chemical Reactions
... formulas of the starting materials and products (which every chemical equation must do) and the physical state of each reactant and product, it does not give the amounts correctly. It is not balanced, which means that the number of atoms on the left side of the equation is not the same as the number ...
... formulas of the starting materials and products (which every chemical equation must do) and the physical state of each reactant and product, it does not give the amounts correctly. It is not balanced, which means that the number of atoms on the left side of the equation is not the same as the number ...
CHAPTER-8 NCERT SOLUTIONS
... Adding the two half reactions, we have the net balanced redox reaction as: (b)Following the steps as in part (a), we have the oxidation half reaction as: And the reduction half reaction as: Multiplying the oxidation half reaction by 5 and the reduction half reaction by 2, and then by adding them, we ...
... Adding the two half reactions, we have the net balanced redox reaction as: (b)Following the steps as in part (a), we have the oxidation half reaction as: And the reduction half reaction as: Multiplying the oxidation half reaction by 5 and the reduction half reaction by 2, and then by adding them, we ...
Chapter 04
... To determine the molecular, ionic and net ionic equations: 1) Write and balance the molecular equation, predicting the products by assuming that the cations trade anions. 2) Write the ionic equation by separating strong electrolytes into their constituent ions. 3) Write the net ionic equation by ide ...
... To determine the molecular, ionic and net ionic equations: 1) Write and balance the molecular equation, predicting the products by assuming that the cations trade anions. 2) Write the ionic equation by separating strong electrolytes into their constituent ions. 3) Write the net ionic equation by ide ...
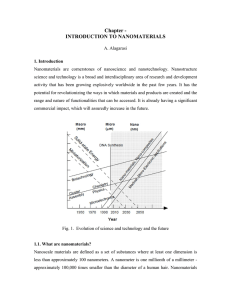
Chapter - INTRODUCTION TO NANOMATERIALS
... catalytic, electric, magnetic, optical and electronic functions. The production of nanophase or cluster-assembled materials is usually based upon the creation of separated small clusters which then are fused into a bulk-like material or on their embedding into compact liquid or solid matrix material ...
... catalytic, electric, magnetic, optical and electronic functions. The production of nanophase or cluster-assembled materials is usually based upon the creation of separated small clusters which then are fused into a bulk-like material or on their embedding into compact liquid or solid matrix material ...
Molarity = M (Concentration of Solutions)
... Later R = 8.314 J / (mol K) = 8.314 J mol-1 K-1 An ideal gas is one for which both the volume of molecules and forces between the molecules are so small that they have insignificant effect on its P-V-T behavior. Independent of substance, in the limit that n/V →0, all gases behave ideally. Usually tr ...
... Later R = 8.314 J / (mol K) = 8.314 J mol-1 K-1 An ideal gas is one for which both the volume of molecules and forces between the molecules are so small that they have insignificant effect on its P-V-T behavior. Independent of substance, in the limit that n/V →0, all gases behave ideally. Usually tr ...
Insertion of SO2 into the Metal−Carbon Bonds of Rhodium and
... able to cleanly insert SO2 into their M-C bonds. Both are based on group VIII metal complexes containing an aryl or alkyl ligand. The reactivities of the SO2-inserted species have been studied, and we have shown that it is possible to release the RSO2 group from the metal to form sulfinic acids. Res ...
... able to cleanly insert SO2 into their M-C bonds. Both are based on group VIII metal complexes containing an aryl or alkyl ligand. The reactivities of the SO2-inserted species have been studied, and we have shown that it is possible to release the RSO2 group from the metal to form sulfinic acids. Res ...
Chapter 4: Solution Chemistry: The Hydrosphere
... a. __________________________ and __________________________ b. __________________________ and __________________________ c. __________________________ and __________________________ Note: H2O is amphoteric since it can behave as an acid or a base. CHEM 161: Chapter 4 v0916 ...
... a. __________________________ and __________________________ b. __________________________ and __________________________ c. __________________________ and __________________________ Note: H2O is amphoteric since it can behave as an acid or a base. CHEM 161: Chapter 4 v0916 ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.