A GREEN APPROACH FOR THE SELECTIVE REDUCTION OF
... Aromatic carbonyl compounds are receiving considerable attention due to their importance as versatile intermediates in the construction of biologically active structural motifs, as well as to their status of main constituents of many natural products [1 – 3]. Although the Ni-Al alloy in aqueous alka ...
... Aromatic carbonyl compounds are receiving considerable attention due to their importance as versatile intermediates in the construction of biologically active structural motifs, as well as to their status of main constituents of many natural products [1 – 3]. Although the Ni-Al alloy in aqueous alka ...
File
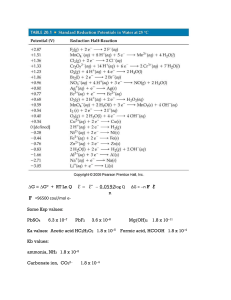
... ______1. Which of the following will increase the Ksp of PbCl2 ? A) Addition of HCl to the solution B) Addition of Pb(NO3)2 to the solution C) An increase in temperature D) All of these. ______2. AgCl would be LEAST soluble in a solution of 1.00 molar A) HNO3 B) AgNO3 C) HCl D) BaCl2 ______3. Methan ...
... ______1. Which of the following will increase the Ksp of PbCl2 ? A) Addition of HCl to the solution B) Addition of Pb(NO3)2 to the solution C) An increase in temperature D) All of these. ______2. AgCl would be LEAST soluble in a solution of 1.00 molar A) HNO3 B) AgNO3 C) HCl D) BaCl2 ______3. Methan ...
Practice Exam 4
... Ne and Ar are both atoms so they should have less entropy than a molecular substance, which has more complexity. Ar will have a higher entropy than Ne because it has a larger mass and more fundamental particles. The correct order is H2 O(ℓ) < Ne(g) < Ar(g) < CO2 (g). 017 10.0 points Consider the fol ...
... Ne and Ar are both atoms so they should have less entropy than a molecular substance, which has more complexity. Ar will have a higher entropy than Ne because it has a larger mass and more fundamental particles. The correct order is H2 O(ℓ) < Ne(g) < Ar(g) < CO2 (g). 017 10.0 points Consider the fol ...
Example 1-2
... The solubility of a solute is the amount that can be dissolved in a given quantity of solvent at a given temperature. For example, the solubility of lead (II) nitrate is 56 g/100 mL at 20oC. The solubilities of ionic solids in water vary over a wide range of values. For convenience, we divide compou ...
... The solubility of a solute is the amount that can be dissolved in a given quantity of solvent at a given temperature. For example, the solubility of lead (II) nitrate is 56 g/100 mL at 20oC. The solubilities of ionic solids in water vary over a wide range of values. For convenience, we divide compou ...
UNIVERSITY OF TARTU THE GIFTED AND
... formed were collected into two upside-down beakers that were filled with water. In one beaker, the amount of water displaced by the gas was two times the amount displaced in the other beaker. a) Write the equations for the half-reactions for both the anode and cathode. Write the equation for the ov ...
... formed were collected into two upside-down beakers that were filled with water. In one beaker, the amount of water displaced by the gas was two times the amount displaced in the other beaker. a) Write the equations for the half-reactions for both the anode and cathode. Write the equation for the ov ...
File
... The Data Booklet value for the enthalpy of combustion of methanol is –726 kJ mol–1. Suggest why this value differs from the values calculated in parts (a) and (b). (i) ...
... The Data Booklet value for the enthalpy of combustion of methanol is –726 kJ mol–1. Suggest why this value differs from the values calculated in parts (a) and (b). (i) ...
Chapter 3: Calculations with Chemical Formulas
... Free elements are assigned an oxidation state of zero. The sum of the oxidation states of all that atoms in a species must be equal to the net charge on the species. The alkali metals (Li, Na, K, Rb, and Cs) in compounds are always assigned an oxidation state of +1. Fluorine in compounds is always a ...
... Free elements are assigned an oxidation state of zero. The sum of the oxidation states of all that atoms in a species must be equal to the net charge on the species. The alkali metals (Li, Na, K, Rb, and Cs) in compounds are always assigned an oxidation state of +1. Fluorine in compounds is always a ...
Document
... Elements can exist in more than one physical state, and some elements exist in more than one distinct form in the same physical state. For example, carbon can exist as graphite or as diamond; oxygen can exist as O2 or as O3 (ozone). These different forms of an element in the same physical state are ...
... Elements can exist in more than one physical state, and some elements exist in more than one distinct form in the same physical state. For example, carbon can exist as graphite or as diamond; oxygen can exist as O2 or as O3 (ozone). These different forms of an element in the same physical state are ...
Acid-Base Reactions Worksheet #2 - Mro
... large quantities that it flows down the body and drips onto the ground. Since the purpose of perspiration is to produce a cooling effect by evaporation, why does the human body not produce just enough perspiration to keep the skin surface moist? The production of perspiration requires relatively lar ...
... large quantities that it flows down the body and drips onto the ground. Since the purpose of perspiration is to produce a cooling effect by evaporation, why does the human body not produce just enough perspiration to keep the skin surface moist? The production of perspiration requires relatively lar ...
Oxidation numbers
... In fact, oxidation never takes place on its own - nor does reduction. When one substance is oxidised in a reaction, another one is reduced. A Redox reaction is one in which both reduction and oxidation take place. To work out which element is oxidised and which is reduced in a reaction, we go throug ...
... In fact, oxidation never takes place on its own - nor does reduction. When one substance is oxidised in a reaction, another one is reduced. A Redox reaction is one in which both reduction and oxidation take place. To work out which element is oxidised and which is reduced in a reaction, we go throug ...
Determination of Cystein and Methionine by Oscillating Chemical
... Mechanisms for oscillatory chemical systems have been pursued almost as soon as chemists finally recognized the possibility of oscillation in a homogeneous medium [17–21]. Understanding why and how such complicated behavior arises in terms of chemical species and the interaction among them is a fasc ...
... Mechanisms for oscillatory chemical systems have been pursued almost as soon as chemists finally recognized the possibility of oscillation in a homogeneous medium [17–21]. Understanding why and how such complicated behavior arises in terms of chemical species and the interaction among them is a fasc ...
Standard - Santee Education Complex
... other and their atoms become joined. The electrons that interact with each other are VALENCE ELECTRONS, the ones that reside in the outermost electron shell of an atom. There are two main types of bonding discussed here. A COVALENT BOND results when two atoms "share" valence electrons between them. ...
... other and their atoms become joined. The electrons that interact with each other are VALENCE ELECTRONS, the ones that reside in the outermost electron shell of an atom. There are two main types of bonding discussed here. A COVALENT BOND results when two atoms "share" valence electrons between them. ...
1.8 Thermodynamics
... The entropy contribution depends on temperature, T (K) at which the reaction takes place. TDS ...
... The entropy contribution depends on temperature, T (K) at which the reaction takes place. TDS ...
Module 2 Alcohols, halogenoalkanes and analysis
... Throughout the centuries, chemists have synthesised new substances and investigated their properties in the search for more useful materials. In the recent past, organic chemists have developed a broad range of original and exciting materials, such as pharmaceuticals, refrigerants, solvents and plas ...
... Throughout the centuries, chemists have synthesised new substances and investigated their properties in the search for more useful materials. In the recent past, organic chemists have developed a broad range of original and exciting materials, such as pharmaceuticals, refrigerants, solvents and plas ...
SOL Review Part 3 Nomenclature reactions
... iron and 1 liter of 3M H2SO4 at STP. Which reaction will go to completion first and why? A Beaker A because of increased surface area B Beaker B because of increased surface area C Beaker A because of a higher concentration level D Beaker B because of a higher concentration level ...
... iron and 1 liter of 3M H2SO4 at STP. Which reaction will go to completion first and why? A Beaker A because of increased surface area B Beaker B because of increased surface area C Beaker A because of a higher concentration level D Beaker B because of a higher concentration level ...
ap chemistry 2005/2006
... 3-4 days of lecture focused on the key objectives listed in the syllabus, including teacher demonstrations 1-2 days of lab activity. Labs may exceed one 90 minute class, depending on the requirements of the specific lab activity. In addition, some sections/objectives are more conducive to lab ac ...
... 3-4 days of lecture focused on the key objectives listed in the syllabus, including teacher demonstrations 1-2 days of lab activity. Labs may exceed one 90 minute class, depending on the requirements of the specific lab activity. In addition, some sections/objectives are more conducive to lab ac ...
2015 Dr. Jay L. Wile, All rights reserved.
... oxygen to make 49.9 grams of a gas, along with some leftover oxygen. Are the two gases he made the same? ...
... oxygen to make 49.9 grams of a gas, along with some leftover oxygen. Are the two gases he made the same? ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.