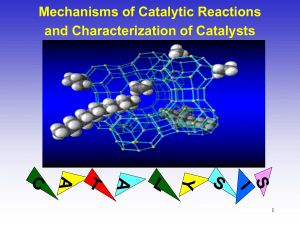
Catalysis and Catalyst
... For the Eley-Rideal mechanism: the rate will increase with increasing coverage until the surface is completely covered by A*. For the Langmuir-Hinshelwood mechanism: the rate will go through a maximum and end up at zero, when the surface is completely covered by A*. This happens because the step ...
... For the Eley-Rideal mechanism: the rate will increase with increasing coverage until the surface is completely covered by A*. For the Langmuir-Hinshelwood mechanism: the rate will go through a maximum and end up at zero, when the surface is completely covered by A*. This happens because the step ...
Nugget
... David E. Lewis, Department of Chemistry, University of Wisconsin-Eau Claire, Eau Claire, WI 54702 The Tröger’s base skeleton is a rigid framework containing two chiral nitrogen atoms at bridgehead positions. Under acid catalysis, the ring system undergoes inversion, but two mechanisms for the invers ...
... David E. Lewis, Department of Chemistry, University of Wisconsin-Eau Claire, Eau Claire, WI 54702 The Tröger’s base skeleton is a rigid framework containing two chiral nitrogen atoms at bridgehead positions. Under acid catalysis, the ring system undergoes inversion, but two mechanisms for the invers ...
Notes on Chapter 12 Chemical Equilibrium
... 2. Reaction rates = speed of the reaction = change in concentration/change in time which can be measured in terms of formation of product or loss of reactants over time. Factors that affect the rate of reaction: a. temperature- increase in temperature increases reaction rates b. concentration- incre ...
... 2. Reaction rates = speed of the reaction = change in concentration/change in time which can be measured in terms of formation of product or loss of reactants over time. Factors that affect the rate of reaction: a. temperature- increase in temperature increases reaction rates b. concentration- incre ...
Reactions
... • Chemical reaction – a process in which bonds of compounds are broken and reformed into different compounds • Molecules are rearranged during reactions, but composition stays the same • There are the same number of atoms in the products as there are in the reactants • Reactant – the starting materi ...
... • Chemical reaction – a process in which bonds of compounds are broken and reformed into different compounds • Molecules are rearranged during reactions, but composition stays the same • There are the same number of atoms in the products as there are in the reactants • Reactant – the starting materi ...
Equilibrium - Cobb Learning
... more quickly. • To make a flameless heater, magnesium dust is mixed with salt and a little iron dust in a thin, flexible pad about the size of a playing card. • To activate the heater, a soldier adds a little water. Within seconds the flameless heater reaches the boiling point and is bubbling and st ...
... more quickly. • To make a flameless heater, magnesium dust is mixed with salt and a little iron dust in a thin, flexible pad about the size of a playing card. • To activate the heater, a soldier adds a little water. Within seconds the flameless heater reaches the boiling point and is bubbling and st ...
Subject:
... affects the progression of equilibrium in a reversible reaction Learning Targets: (“I can” or “I will” statements) I will understand the factors that affect the rate of reactions I will be able to define catalyst and understand their role in the rate of chemical reactions. I will be able to interpre ...
... affects the progression of equilibrium in a reversible reaction Learning Targets: (“I can” or “I will” statements) I will understand the factors that affect the rate of reactions I will be able to define catalyst and understand their role in the rate of chemical reactions. I will be able to interpre ...
Project Details PPT
... oP R E V E N T W A S T E B Y U S I N G A SOLVENTLESS PROCESS. oM I N I M I Z E A M O U N T S O F S O L V E N T S A N D REAGENTS USED. ...
... oP R E V E N T W A S T E B Y U S I N G A SOLVENTLESS PROCESS. oM I N I M I Z E A M O U N T S O F S O L V E N T S A N D REAGENTS USED. ...
Title - Iowa State University
... 3. Which of the following statements about catalysts is false? a. A catalyst will speed up the rate of a reaction. b. Catalysts are used in very many commercially important chemical reactions. c. Catalytic converters are examples of heterogeneous catalysts. d. A catalyst can cause a nonspontaneous r ...
... 3. Which of the following statements about catalysts is false? a. A catalyst will speed up the rate of a reaction. b. Catalysts are used in very many commercially important chemical reactions. c. Catalytic converters are examples of heterogeneous catalysts. d. A catalyst can cause a nonspontaneous r ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.