Particles - Townley Grammar School
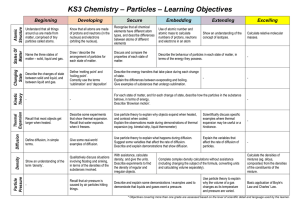
... Explain the differences between evaporating and boiling. Give examples of substances that undergo sublimation. ...
... Explain the differences between evaporating and boiling. Give examples of substances that undergo sublimation. ...
Name:_____________ Chemistry 114 Second Hour Exam
... HF will form intermolecular hydrogen bonds, HCl won’t; this will give HF stronger intermolecular interactions and give it a higher boiling point. I2 has a lower vapor pressure than Cl2 at room temperature. I2 is larger that Cl2, this will make its London force larger to give it stronger intermolecul ...
... HF will form intermolecular hydrogen bonds, HCl won’t; this will give HF stronger intermolecular interactions and give it a higher boiling point. I2 has a lower vapor pressure than Cl2 at room temperature. I2 is larger that Cl2, this will make its London force larger to give it stronger intermolecul ...
Atomic arrangement, short and long range order, point. Direction
... longrange order. In an ideal gas the arrangementof an atom at any point in space is independent of the arrangement of other atoms. Thus, bothlong-range and shortrange order are absent in the ideal gas, but liquids and amorphous solidsexhibit sho rt-rangeordera certain regularity in the arrangement o ...
... longrange order. In an ideal gas the arrangementof an atom at any point in space is independent of the arrangement of other atoms. Thus, bothlong-range and shortrange order are absent in the ideal gas, but liquids and amorphous solidsexhibit sho rt-rangeordera certain regularity in the arrangement o ...
Lab Equipment notes powerpoint
... Burner or “Bunsen burner” has a flame for heating substances Striker has flint in it, for starting the flame Orange rubber tubing carries gas from the gas jets to the burner ...
... Burner or “Bunsen burner” has a flame for heating substances Striker has flint in it, for starting the flame Orange rubber tubing carries gas from the gas jets to the burner ...
Course Pack3 Phase Diagrams
... ∆Hsoln is (+) for NaCl in H2O ∆Hsoln is (–) for Na2SO4 in H2O ∆Hsoln is (–) for O2 in H2O Consider the case that ∆Hmix is negative: since ∆Smix is positive then ∆Gsoln will have to be negative and the reaction happens. Now consider the case that ∆Hmix is positive: in this case the spontaneity of the ...
... ∆Hsoln is (+) for NaCl in H2O ∆Hsoln is (–) for Na2SO4 in H2O ∆Hsoln is (–) for O2 in H2O Consider the case that ∆Hmix is negative: since ∆Smix is positive then ∆Gsoln will have to be negative and the reaction happens. Now consider the case that ∆Hmix is positive: in this case the spontaneity of the ...
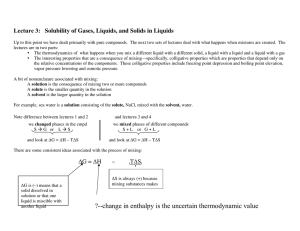
Lecture 3: Solubility of Gases, Liquids, and Solids in Liquids ΔG
... These compete and we want solvation effects H H O ------- ...
... These compete and we want solvation effects H H O ------- ...
Real Gases
... etc. As mentioned, this process can be inverted and we can solve for B'(T), C'(T), etc. in terms of B(T), C(T), etc. This gives: B'(T) = C'(T) = etc. Finally, real gases also differ from an Ideal gas in that if cooled or compressed, they will typically condense into a liquid. This behavior can be il ...
... etc. As mentioned, this process can be inverted and we can solve for B'(T), C'(T), etc. in terms of B(T), C(T), etc. This gives: B'(T) = C'(T) = etc. Finally, real gases also differ from an Ideal gas in that if cooled or compressed, they will typically condense into a liquid. This behavior can be il ...
Physical concept of the surface tension of the liquid until some time
... As a result of calculations for molecules, similar in the symmetric spherical form , the coefficient n must be equal to unity. The processing of the reference data of the thermophysical properties of most substances has confirmed the theoretical formulas with an accuracy of 15%. Some substances with ...
... As a result of calculations for molecules, similar in the symmetric spherical form , the coefficient n must be equal to unity. The processing of the reference data of the thermophysical properties of most substances has confirmed the theoretical formulas with an accuracy of 15%. Some substances with ...
matterLessonPlan
... The smallest unit of matter An atom is does have smaller components (electrons, protons, neutrons, etc). An atom, however, is the smallest particle of an element that still has the chemical properties of that element. Scientists have found 115 types of atoms so far, and new ones are still bein ...
... The smallest unit of matter An atom is does have smaller components (electrons, protons, neutrons, etc). An atom, however, is the smallest particle of an element that still has the chemical properties of that element. Scientists have found 115 types of atoms so far, and new ones are still bein ...
Acids and bases
... For example, the melting point of NaCl is 1073 K, but is lowered if CaCl2 is added as in the Downs process. In the solid state, HgCl2 forms a molecular lattice, and layer structures are adopted by HgBr2 (distorted CdI2 lattice) and HgI2. An important group of molten salts with more convenient operat ...
... For example, the melting point of NaCl is 1073 K, but is lowered if CaCl2 is added as in the Downs process. In the solid state, HgCl2 forms a molecular lattice, and layer structures are adopted by HgBr2 (distorted CdI2 lattice) and HgI2. An important group of molten salts with more convenient operat ...
Chapter 2 What Is Matter
... in a liquid. Since there are three common phases of matter, there are nine possible types of solutions. Such as: Air is a gas dissolved in a gas. Soft drinks are a gas dissolved in a liquid. Humid air is a liquid dissolved in a gas. Antifreeze is a liquid dissolved in a liquid. Saltwater is a solid ...
... in a liquid. Since there are three common phases of matter, there are nine possible types of solutions. Such as: Air is a gas dissolved in a gas. Soft drinks are a gas dissolved in a liquid. Humid air is a liquid dissolved in a gas. Antifreeze is a liquid dissolved in a liquid. Saltwater is a solid ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI –600 034 B.Sc., DEGREE EXAMINATION - CHEMISTRY
... 12. Internal energy and enthalpy remain constant in the isothermal expansion of an ideal gas Explain. 13. For the reaction N2 (g) + 3H2 (g) 2 NH3(g). Kp is 1.64 x 10-4 at 673 k. Calculate G when the partial pressure of N2, H2 and NH3 are 10 atm, 30 atm and 3 atm respectively. Is the reaction spon ...
... 12. Internal energy and enthalpy remain constant in the isothermal expansion of an ideal gas Explain. 13. For the reaction N2 (g) + 3H2 (g) 2 NH3(g). Kp is 1.64 x 10-4 at 673 k. Calculate G when the partial pressure of N2, H2 and NH3 are 10 atm, 30 atm and 3 atm respectively. Is the reaction spon ...
Matter Notes
... For a pure substance a change of state occurs at a constant temperature (distinct physical properties). 2. Freezing pt: a constant temp at which a pure substance changes from a liquid to solid 3. Melting pt: a constant temp at which . . . solid to liquid note: at the freezing and melting point – sol ...
... For a pure substance a change of state occurs at a constant temperature (distinct physical properties). 2. Freezing pt: a constant temp at which a pure substance changes from a liquid to solid 3. Melting pt: a constant temp at which . . . solid to liquid note: at the freezing and melting point – sol ...
Mr Alasdair Ross at Southpointe Academy
... The passage of molecules directly from the solid state to the vapour state is called sublimation. The reverse process is called deposition. A dynamic equilibrium is reached when the rates of sublimation and deposition become equal. Like vapour in equilibrium with a liquid, vapour in equilibrium with ...
... The passage of molecules directly from the solid state to the vapour state is called sublimation. The reverse process is called deposition. A dynamic equilibrium is reached when the rates of sublimation and deposition become equal. Like vapour in equilibrium with a liquid, vapour in equilibrium with ...
The nature of matter
... pressure – a case for atoms Pumping up a tire increases the number of molecules Pressure is caused by the energetic molecules striking the tire wall More molecules – higher pressure Higher temperature – higher pressure ...
... pressure – a case for atoms Pumping up a tire increases the number of molecules Pressure is caused by the energetic molecules striking the tire wall More molecules – higher pressure Higher temperature – higher pressure ...
OCR Document - Northern Highlands
... b. The pill dissolves in water. c. Cutting the pill into 2 pieces. d. Mixing the pill in ice cream to make it easier to swallow. 38. A_____________ shows all states of matter of a substance and the temperatures at which they change state. a. equilibrium curve b. evaporation curve c. condensation dia ...
... b. The pill dissolves in water. c. Cutting the pill into 2 pieces. d. Mixing the pill in ice cream to make it easier to swallow. 38. A_____________ shows all states of matter of a substance and the temperatures at which they change state. a. equilibrium curve b. evaporation curve c. condensation dia ...
The Physical Properties And Physical Changes of Substances
... which are not foreseen when they are proposed because they provide explanations for entire “fields” of related behaviour. • Theories are sometimes called models because they often provide a concrete way to examine, predict, and test the workings of nature. • A theory cannot be “proven” but it may ha ...
... which are not foreseen when they are proposed because they provide explanations for entire “fields” of related behaviour. • Theories are sometimes called models because they often provide a concrete way to examine, predict, and test the workings of nature. • A theory cannot be “proven” but it may ha ...
B. The Physical Properties of Matter
... which are not foreseen when they are proposed because they provide explanations for entire “fields” of related behaviour. Theories are sometimes called models because they often provide a concrete way to examine, predict, and test the workings of nature. A theory cannot be “proven” but it may ha ...
... which are not foreseen when they are proposed because they provide explanations for entire “fields” of related behaviour. Theories are sometimes called models because they often provide a concrete way to examine, predict, and test the workings of nature. A theory cannot be “proven” but it may ha ...
worksheer format 11-12
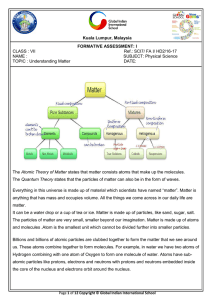
... The Atomic Theory of Matter states that matter consists atoms that make up the molecules. The Quantum Theory states that the particles of matter can also be in the form of waves. Everything in this universe is made up of material which scientists have named “matter”. Matter is anything that has mass ...
... The Atomic Theory of Matter states that matter consists atoms that make up the molecules. The Quantum Theory states that the particles of matter can also be in the form of waves. Everything in this universe is made up of material which scientists have named “matter”. Matter is anything that has mass ...
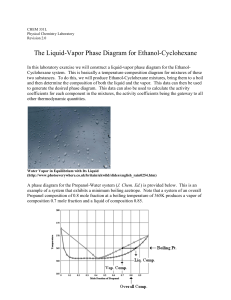
The Liquid-Vapor Phase Diagram for Ethanol
... Once A,az and B,az have been determined, the van Laar coefficients AL and BL can be calculated from equations (14) and (15). Now equations (12) and (13) can be used to calculate model values for A and B at any other system composition. These model coefficients can then be used to model the entir ...
... Once A,az and B,az have been determined, the van Laar coefficients AL and BL can be calculated from equations (14) and (15). Now equations (12) and (13) can be used to calculate model values for A and B at any other system composition. These model coefficients can then be used to model the entir ...
Chapter 6 - Department of Chemical Engineering
... More than often, mixtures consisting of at least two components form an azeotrope, which occurs when liquid and vapor fractions cannot be changed during distillation [2]. An azeotrope can result in two conditions, where the mixture can boil at either higher or lower temperature than the boiling poin ...
... More than often, mixtures consisting of at least two components form an azeotrope, which occurs when liquid and vapor fractions cannot be changed during distillation [2]. An azeotrope can result in two conditions, where the mixture can boil at either higher or lower temperature than the boiling poin ...
Chemistry in Focus: Tiny Thermometers
... trying to make tiny (nanoscale) gallium nitride wires. However, when they examined the results of their experiment, they discovered tiny tubes of carbon atoms that were filled with elemental gallium. Because gallium is a liquid over an unusually large temperature range, it makes a perfect working li ...
... trying to make tiny (nanoscale) gallium nitride wires. However, when they examined the results of their experiment, they discovered tiny tubes of carbon atoms that were filled with elemental gallium. Because gallium is a liquid over an unusually large temperature range, it makes a perfect working li ...
Ideal gas
... the square root of its density. Combined with Avogadro's law (i.e. since equal volumes have equal number of molecules) this is the same as being inversely proportional to the root of the molecular weight. ...
... the square root of its density. Combined with Avogadro's law (i.e. since equal volumes have equal number of molecules) this is the same as being inversely proportional to the root of the molecular weight. ...
Matter Exam Study Guide
... Directions: Complete the study guide to receive 5 bonus points on your exam. This must be turned in complete for credit. Please put your answers on a separate piece of paper. 1. What is the definition of matter? 2. What is the definition of mass? 3. What is the definition of volume? 4. What is the d ...
... Directions: Complete the study guide to receive 5 bonus points on your exam. This must be turned in complete for credit. Please put your answers on a separate piece of paper. 1. What is the definition of matter? 2. What is the definition of mass? 3. What is the definition of volume? 4. What is the d ...
Liquid

A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, and plasma), and is the only state with a definite volume but no fixed shape. A liquid is made up of tiny vibrating particles of matter, such as atoms, held together by intermolecular bonds. Water is, by far, the most common liquid on Earth. Like a gas, a liquid is able to flow and take the shape of a container. Most liquids resist compression, although others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly constant density. A distinctive property of the liquid state is surface tension, leading to wetting phenomena.The density of a liquid is usually close to that of a solid, and much higher than in a gas. Therefore, liquid and solid are both termed condensed matter. On the other hand, as liquids and gases share the ability to flow, they are both called fluids. Although liquid water is abundant on Earth, this state of matter is actually the least common in the known universe, because liquids require a relatively narrow temperature/pressure range to exist. Most known matter in the universe is in gaseous form (with traces of detectable solid matter) as interstellar clouds or in plasma form within stars.