Theory for an order-driven disruption of the liquid state in water
... Water is known to exhibit a number of peculiar physical properties because of the strong orientational dependence of the intermolecular hydrogen bonding interactions that dominate its liquid state. Recent full-atom simulations of water in a nanolayer between graphite plates submersed in an aqueous m ...
... Water is known to exhibit a number of peculiar physical properties because of the strong orientational dependence of the intermolecular hydrogen bonding interactions that dominate its liquid state. Recent full-atom simulations of water in a nanolayer between graphite plates submersed in an aqueous m ...
Export To Word
... This is free, easy-to-read, and accessible book that explains the three states of matter. The book may be downloaded as slide Three Kinds of Matter: Solids, show in PowerPoint, Impress, or Flash format. The book can be Liquids, and Gases speech enabled and accessed using multiple interfaces, includi ...
... This is free, easy-to-read, and accessible book that explains the three states of matter. The book may be downloaded as slide Three Kinds of Matter: Solids, show in PowerPoint, Impress, or Flash format. The book can be Liquids, and Gases speech enabled and accessed using multiple interfaces, includi ...
Concentration of solutions
... move through a GC column as gases. The compounds partition between a stationary phase, which can be either solid or liquid, and a mobile phase (gas). The differential partitioning into the stationary phase allows the compounds to be separated in time and space. http://www.cee.vt.edu/ewr/environmenta ...
... move through a GC column as gases. The compounds partition between a stationary phase, which can be either solid or liquid, and a mobile phase (gas). The differential partitioning into the stationary phase allows the compounds to be separated in time and space. http://www.cee.vt.edu/ewr/environmenta ...
Soquids Answers M/C 58. C 68. C 27. E 54. A 21. A 49. C 50. B 51
... (b) (i) a solution made from a non-volatile solute has a higher boiling point than the pure solvent because the solution has a lower vapor pressure than the water (Raoult’s Law) . the temperature of the solution has be higher to produce enough vapor pressure to equal the atmospheric pressure (i.e., ...
... (b) (i) a solution made from a non-volatile solute has a higher boiling point than the pure solvent because the solution has a lower vapor pressure than the water (Raoult’s Law) . the temperature of the solution has be higher to produce enough vapor pressure to equal the atmospheric pressure (i.e., ...
Generation of 3-Dimensionally Integrated Micro Solution Plasmas and Its Application to Decomposition of Organic Contaminants in Water
... 2.1 Dependence on electrical conductivity of liquid Figure 2(b) shows electric field in the bubble indicated by “Probe point” in Fig. 1. The electric field in the bubble reaches its maximum value around 10-7 - 10-6 s after applying the ramp voltage. Since the dielectric framework and gas bubbles are ...
... 2.1 Dependence on electrical conductivity of liquid Figure 2(b) shows electric field in the bubble indicated by “Probe point” in Fig. 1. The electric field in the bubble reaches its maximum value around 10-7 - 10-6 s after applying the ramp voltage. Since the dielectric framework and gas bubbles are ...
Density In Class Assignment
... cylinder. The mass of the empty graduated cylinder is found to be 40.57 g. The mass of the graduated cylinder and the liquid is recorded as 105.37 g. The volume of the liquid is read and recorded as 54.0 cm3. What is the density of the liquid? ...
... cylinder. The mass of the empty graduated cylinder is found to be 40.57 g. The mass of the graduated cylinder and the liquid is recorded as 105.37 g. The volume of the liquid is read and recorded as 54.0 cm3. What is the density of the liquid? ...
Dangerous Goods - `OnGuard®` Safety Training
... Because corrosives may react with non-DG goods or corrode them or their packagings, the corrosives storage area should not be used to store any other dangerous goods. DG licensing is required if the total quantity on site exceeds: * 50 kg/L of PG I * 500 kg/L of PG 11 * 1,000 kg/L of PG 111 Storage ...
... Because corrosives may react with non-DG goods or corrode them or their packagings, the corrosives storage area should not be used to store any other dangerous goods. DG licensing is required if the total quantity on site exceeds: * 50 kg/L of PG I * 500 kg/L of PG 11 * 1,000 kg/L of PG 111 Storage ...
n - UCL Department of Electronic and Electrical Engineering
... • Lyotropic phases occur for molecules dissolved in a solution • Different phases occur with concentration • Often the solvent is water and the molecules have an hydrophilic end and an hydrophobic end (e.g. detergents with polar (hydrophilic) and non-polar ...
... • Lyotropic phases occur for molecules dissolved in a solution • Different phases occur with concentration • Often the solvent is water and the molecules have an hydrophilic end and an hydrophobic end (e.g. detergents with polar (hydrophilic) and non-polar ...
Experimental and Simulation Results for the Removal of H2S from
... to the packing surface. When hydrogen sulfide is absorbed into a sodium hydroxide solution, it can react directly with hydroxyl ions by a proton transfer reaction as seen in reaction R.6 (Section III F). Compared to the diffusion phenomena, this reaction is extremely rapid and can be considered inst ...
... to the packing surface. When hydrogen sulfide is absorbed into a sodium hydroxide solution, it can react directly with hydroxyl ions by a proton transfer reaction as seen in reaction R.6 (Section III F). Compared to the diffusion phenomena, this reaction is extremely rapid and can be considered inst ...
Enliven your palate while relishing all-day flavors.
... of California to cause birth defects or other reproductive harm. Paisley Vapor products are not smoking cessation products and have not been evaluated by the Food and Drug Administration, nor are they intended to treat, prevent or cure any disease or condition. ...
... of California to cause birth defects or other reproductive harm. Paisley Vapor products are not smoking cessation products and have not been evaluated by the Food and Drug Administration, nor are they intended to treat, prevent or cure any disease or condition. ...
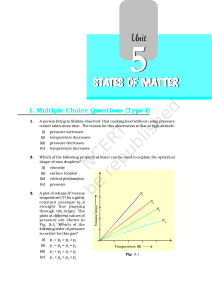
states of matter
... what will be the state of the substance? 53. Why does sharp glass edge become smooth on heating it upto its melting point in a flame? Explain which property of liquids is responsible for this phenomenon. ...
... what will be the state of the substance? 53. Why does sharp glass edge become smooth on heating it upto its melting point in a flame? Explain which property of liquids is responsible for this phenomenon. ...
Latent Heat of Vaporization and Speci c Heat - Physlab
... in degrees kelvin. Each direction in which a particle is free to move is counted as a degree of freedom. For example if a particle can only move along the x axis, it has 1 degree of freedom. This principle of equal sharing of energy between the degrees of freedom is called the principle of equiparti ...
... in degrees kelvin. Each direction in which a particle is free to move is counted as a degree of freedom. For example if a particle can only move along the x axis, it has 1 degree of freedom. This principle of equal sharing of energy between the degrees of freedom is called the principle of equiparti ...
CHAPTER 1-MATTER AND ITS PROPERTIES The
... used in ____the paper industry____ 18. In order to seperate iron powder and pellicle components from a mixture we use the method of ____Floating and Precipitation______ 19. If a heterogenous mixture comprises solid and liquid components with different densities, than a further precipitation process ...
... used in ____the paper industry____ 18. In order to seperate iron powder and pellicle components from a mixture we use the method of ____Floating and Precipitation______ 19. If a heterogenous mixture comprises solid and liquid components with different densities, than a further precipitation process ...
Journal of Molecular Catalysis A: Chemical Enhancing
... irradiation time. Resulting mixture was allowed to cool, followed by extraction with diethyl ether (3 × 10 mL). Combined organic layer was washed with brine and dried over anhydrous sodium sulfate. Evaporation of the solvent under reduced pressure afforded the crude product that was purified by colum ...
... irradiation time. Resulting mixture was allowed to cool, followed by extraction with diethyl ether (3 × 10 mL). Combined organic layer was washed with brine and dried over anhydrous sodium sulfate. Evaporation of the solvent under reduced pressure afforded the crude product that was purified by colum ...
Nemcova abstract- ICPIG.rtf - Queen`s University Belfast
... electrode circular device with a coaxial earth and with each electrode driven with 100kHz RF bipolar square wave voltage. The time dependent currentvoltage characteristics are measured for one of the electrodes and in the power calculation it is assumed that the characteristics of all four electrode ...
... electrode circular device with a coaxial earth and with each electrode driven with 100kHz RF bipolar square wave voltage. The time dependent currentvoltage characteristics are measured for one of the electrodes and in the power calculation it is assumed that the characteristics of all four electrode ...
AP Lab - MW of Volatile Liquid - North Allegheny School District
... 2. Was the vapor you investigated really "ideal" in the experiment (was it at a temperature that put it far above its boiling point)? Explain the difference between an ideal and non-ideal gas. 3. When placed in the ice bath, did all of the vapor within the flask condense into a liquid? Explain your ...
... 2. Was the vapor you investigated really "ideal" in the experiment (was it at a temperature that put it far above its boiling point)? Explain the difference between an ideal and non-ideal gas. 3. When placed in the ice bath, did all of the vapor within the flask condense into a liquid? Explain your ...
PowerPoint Chapter 14 - Preparatory Chemistry
... • The rate of evaporation is the number of particles moving from liquid to gas per second. • It is dependent on the following: – Surface area of the liquid – Strength of attractions between the particles in the liquid – Temperature ...
... • The rate of evaporation is the number of particles moving from liquid to gas per second. • It is dependent on the following: – Surface area of the liquid – Strength of attractions between the particles in the liquid – Temperature ...
High pressure differential scanning calorimetry of the hydrothermal
... obtained from experiments in the cooling mode showed that the precipitation of the solid salt is kinetically hindered when the measurement is performed in the heating mode and that substantial superheating occurs. Hence, the samples were remeasured in the cooling mode, showing much lower transition ...
... obtained from experiments in the cooling mode showed that the precipitation of the solid salt is kinetically hindered when the measurement is performed in the heating mode and that substantial superheating occurs. Hence, the samples were remeasured in the cooling mode, showing much lower transition ...
Solubility Main article: Solvation The ability of one compound to
... increasing solute concentration. At some point the energy loss outweighs the entropy gain, and no more solute particles can be dissolved; the solution is said to be saturated. However, the point at which a solution can become saturated can change significantly with different environmental factors, s ...
... increasing solute concentration. At some point the energy loss outweighs the entropy gain, and no more solute particles can be dissolved; the solution is said to be saturated. However, the point at which a solution can become saturated can change significantly with different environmental factors, s ...
Introduction
... parameters for ions and solvents [3]. Pure parameters for ammonia are determined from vapor-liquid data. Pure parameters for water are found in the literature [1]. For ammonia-sodium hydroxide-water mixture parameters are fitted with vapor liquid equilibrium data. Experiments The methods for the dir ...
... parameters for ions and solvents [3]. Pure parameters for ammonia are determined from vapor-liquid data. Pure parameters for water are found in the literature [1]. For ammonia-sodium hydroxide-water mixture parameters are fitted with vapor liquid equilibrium data. Experiments The methods for the dir ...
Colligative Properties of an Cyclohexane/1
... First, in order to make sure the apparatus, Du Noűy Tensiometer, was calibrated we placed a 100 mg piece of paper on the meter and recorded a measurement. We found that our correction factor was 1.42. This was found by using the equation mg/R; m=mass of paper, R=measurement reading, and g=accelerati ...
... First, in order to make sure the apparatus, Du Noűy Tensiometer, was calibrated we placed a 100 mg piece of paper on the meter and recorded a measurement. We found that our correction factor was 1.42. This was found by using the equation mg/R; m=mass of paper, R=measurement reading, and g=accelerati ...
Carrying Charges
... Experimental chemicals are never tasted. After each use the well plates must be carefully rinsed with distilled water. Even a little bit of salt or salt water will cause confusing results. Make sure the participants do not mix up the solid chemicals and the spoons used to dispense the chemicals. Con ...
... Experimental chemicals are never tasted. After each use the well plates must be carefully rinsed with distilled water. Even a little bit of salt or salt water will cause confusing results. Make sure the participants do not mix up the solid chemicals and the spoons used to dispense the chemicals. Con ...
Reasoning about Fluids Via Molecular Collections
... about changes in location or phase of MC, when located whithin the realm of influence of the process . For example, the rule associated with liquid-flow implies that when MC is in liquid form in the source, it can move into the path of the flow, and end up in the destination of the flow without chan ...
... about changes in location or phase of MC, when located whithin the realm of influence of the process . For example, the rule associated with liquid-flow implies that when MC is in liquid form in the source, it can move into the path of the flow, and end up in the destination of the flow without chan ...
Liquid

A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, and plasma), and is the only state with a definite volume but no fixed shape. A liquid is made up of tiny vibrating particles of matter, such as atoms, held together by intermolecular bonds. Water is, by far, the most common liquid on Earth. Like a gas, a liquid is able to flow and take the shape of a container. Most liquids resist compression, although others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly constant density. A distinctive property of the liquid state is surface tension, leading to wetting phenomena.The density of a liquid is usually close to that of a solid, and much higher than in a gas. Therefore, liquid and solid are both termed condensed matter. On the other hand, as liquids and gases share the ability to flow, they are both called fluids. Although liquid water is abundant on Earth, this state of matter is actually the least common in the known universe, because liquids require a relatively narrow temperature/pressure range to exist. Most known matter in the universe is in gaseous form (with traces of detectable solid matter) as interstellar clouds or in plasma form within stars.