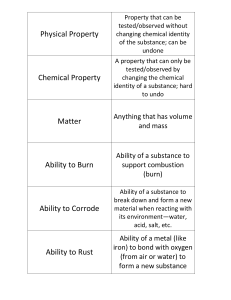
INTRODUCTION There are different ways of classifying materials
... actuators, capacitors, inductors, and electrical insulation. Glass is an amorphous material, derived from molten silica. The fiber optics industry is founded on optical fibers made by using high-purity silica glass. Glasses are also used in houses, cars, computer and television screens, and hundreds ...
... actuators, capacitors, inductors, and electrical insulation. Glass is an amorphous material, derived from molten silica. The fiber optics industry is founded on optical fibers made by using high-purity silica glass. Glasses are also used in houses, cars, computer and television screens, and hundreds ...
Chemistry Learning Goals Chap 14 Solutions Minniear
... hydration, diffusion). SWBAT discuss the factors that determine the rate of solution for a solid solute and a liquid solvent (agitation, temperature and surface area). SWBAT explain the effect of temperature on the solubility of a gas. SWBAT explain the processes involved when a solution has reached ...
... hydration, diffusion). SWBAT discuss the factors that determine the rate of solution for a solid solute and a liquid solvent (agitation, temperature and surface area). SWBAT explain the effect of temperature on the solubility of a gas. SWBAT explain the processes involved when a solution has reached ...
Enter o to this page the details for the document
... 2. Connect the Temperature sensor to an input of the logger. 3. Pour 100 cm3 of water, at about 60oC, into the cup. The volume used will be V1. 4. From the EasySense software’s Home screen select EasyLog. 5. Click on Start to begin logging. 6. Wait until the temperature reaches a maximum (it will ta ...
... 2. Connect the Temperature sensor to an input of the logger. 3. Pour 100 cm3 of water, at about 60oC, into the cup. The volume used will be V1. 4. From the EasySense software’s Home screen select EasyLog. 5. Click on Start to begin logging. 6. Wait until the temperature reaches a maximum (it will ta ...
Lecture 35 (Slides) November 7
... • 1. A total of 3.00 g of liquid water was placed in an evacuated (initially) 12.0L container maintained at a temperature of 80.0 0C. Will a liquid/gas equilibrium be established? What piece of data is required to solve this problem? If no liquid/gas equilibrium is established, determine how much ad ...
... • 1. A total of 3.00 g of liquid water was placed in an evacuated (initially) 12.0L container maintained at a temperature of 80.0 0C. Will a liquid/gas equilibrium be established? What piece of data is required to solve this problem? If no liquid/gas equilibrium is established, determine how much ad ...
2014 Abstract Booklet
... antiferromagnetism. Importantly, rare-earth elements with decreasing ionic radii elevate Tc to as much as 56 K due to the decreasing lattice constant. High-Tc superconductivity in LnFeAsO materials is induced by substituting O ions with F or by creating oxygen vacancies. The complex relationships be ...
... antiferromagnetism. Importantly, rare-earth elements with decreasing ionic radii elevate Tc to as much as 56 K due to the decreasing lattice constant. High-Tc superconductivity in LnFeAsO materials is induced by substituting O ions with F or by creating oxygen vacancies. The complex relationships be ...
Latent Heat of Vaporization and Speci c Heat - Physlab
... We know that molecules are always on the move as they have kinetic energy, but the question is, how is this energy shared? James Clerk Maxwell solved this problem for a large number of molecules. He said that energy is equally divided in all the directions a molecule is free to move. The average ene ...
... We know that molecules are always on the move as they have kinetic energy, but the question is, how is this energy shared? James Clerk Maxwell solved this problem for a large number of molecules. He said that energy is equally divided in all the directions a molecule is free to move. The average ene ...
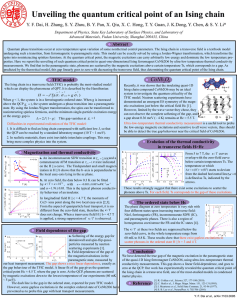
Unveiling the quantum critical point of an Ising chain
... Ultra-low-temperature thermal conductivity measurement is a useful tool to probe the low-energy magnetic excitations and sensitive to all wave vectors, thus should be able to detect the true gap behaviour near the critical field of CoNb2O6. ...
... Ultra-low-temperature thermal conductivity measurement is a useful tool to probe the low-energy magnetic excitations and sensitive to all wave vectors, thus should be able to detect the true gap behaviour near the critical field of CoNb2O6. ...
Fitzroy`s Storm Glass
... The form of storm glasses with which FitzRoy is linked is much different. These chemical storm glasses, also known as storm bottles, are hermetically sealed glass tubes containing a supersaturated mixture of chemicals. They likely appeared around 1750 invented by an alchemist for Italian sailors. T ...
... The form of storm glasses with which FitzRoy is linked is much different. These chemical storm glasses, also known as storm bottles, are hermetically sealed glass tubes containing a supersaturated mixture of chemicals. They likely appeared around 1750 invented by an alchemist for Italian sailors. T ...
Practice Exam 3 - University of Missouri
... C2H2(g) is a. 2 C(s) + H2(g) → C2H2(g) b. 2 C(g) + 2H(g) → C2H2(g) c. 2 C2(g) + 2H(g) → C2H2(g) d. C2H6(g) → C2H2(g) + H2 e. none of the above 8. Which of the following has a standard molar enthalpy of formation of zero at 25° and 1 atm pressure? a. CO2(g) b. H2O(l) c. Zn(s) d. NO(g) ...
... C2H2(g) is a. 2 C(s) + H2(g) → C2H2(g) b. 2 C(g) + 2H(g) → C2H2(g) c. 2 C2(g) + 2H(g) → C2H2(g) d. C2H6(g) → C2H2(g) + H2 e. none of the above 8. Which of the following has a standard molar enthalpy of formation of zero at 25° and 1 atm pressure? a. CO2(g) b. H2O(l) c. Zn(s) d. NO(g) ...
gallium
... Solid gallium has a density of 5.91gcm-3 near room temperature and is one of very few materials that expand on solidification, in this case by 3.1%. This means the metal cannot be stored in glass or metal containers due to the risk of rupture. Other materials with this unusual property are germanium ...
... Solid gallium has a density of 5.91gcm-3 near room temperature and is one of very few materials that expand on solidification, in this case by 3.1%. This means the metal cannot be stored in glass or metal containers due to the risk of rupture. Other materials with this unusual property are germanium ...
OH HO O O
... retained as the strength of the external magnetic field is lowered. This property may permit SMMs to be utilized as components for nano-scale data storage. For any practical application of SMMs , the temperature at w hich the retention of magnetization occurs clearly needs to be raised. Since higher ...
... retained as the strength of the external magnetic field is lowered. This property may permit SMMs to be utilized as components for nano-scale data storage. For any practical application of SMMs , the temperature at w hich the retention of magnetization occurs clearly needs to be raised. Since higher ...
High Performance Computing on Condensed Matter Physics
... often take several hours on 8 CPUs computer. ...
... often take several hours on 8 CPUs computer. ...
What happened to the cup?
... What do you think the effect of cold will have on a plastic cup? Plastics have two temperatures similar to melting and freezing ranges. At the lower point, the glass transition temperature, a flexible plastic becomes rigid like a glass. Check this temperature for the polymer you have identified duri ...
... What do you think the effect of cold will have on a plastic cup? Plastics have two temperatures similar to melting and freezing ranges. At the lower point, the glass transition temperature, a flexible plastic becomes rigid like a glass. Check this temperature for the polymer you have identified duri ...
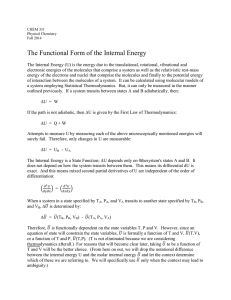
The Functional Form of the Internal Energy
... For this reason, the enthalpy is frequently referred to as the "Heat Function". Its use in thermochemical measurements, where the pressure under which a chemical reaction occurs is constant, should be obvious. Note also that the "natural" state variables for H are T and P; the differential dH as: ...
... For this reason, the enthalpy is frequently referred to as the "Heat Function". Its use in thermochemical measurements, where the pressure under which a chemical reaction occurs is constant, should be obvious. Note also that the "natural" state variables for H are T and P; the differential dH as: ...
Glass transition
The glass–liquid transition or glass transition for short is the reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle state into a molten or rubber-like state. An amorphous solid that exhibits a glass transition is called a glass. Supercooling a viscous liquid into the glass state is called vitrification, from the Latin vitreum, ""glass"" via French vitrifier.Despite the massive change in the physical properties of a material through its glass transition, the transition is not itself a phase transition of any kind; rather it is a laboratory phenomenon extending over a range of temperature and defined by one of several conventions. Such conventions include a constant cooling rate (20 K/min) and a viscosity threshold of 1012 Pa·s, among others. Upon cooling or heating through this glass-transition range, the material also exhibits a smooth step in the thermal-expansion coefficient and in the specific heat, with the location of these effects again being dependent on the history of the material. However, the question of whether some phase transition underlies the glass transition is a matter of continuing research.The glass-transition temperature Tg is always lower than the melting temperature, Tm, of the crystalline state of the material, if one exists.