The Effect of Solvent on a Lewis Acid Catalyzed Diels− Alder
... 1 and 2. In solution, the Singleton-Houk transition structure geometry was altered slightly, where the distance between the Cl and the H3 atoms increased from 2.87 to 2.90 Å. In the second transition structure, Corey’s interaction strengthens by reducing the distance between the Cl atom and the form ...
... 1 and 2. In solution, the Singleton-Houk transition structure geometry was altered slightly, where the distance between the Cl and the H3 atoms increased from 2.87 to 2.90 Å. In the second transition structure, Corey’s interaction strengthens by reducing the distance between the Cl atom and the form ...
Thermal Stability of Mineral-Wool Heat-Insulating
... to carbon(IV) oxide, i.e., to a deep oxidation with the maximum thermal effect at 502°C, nearly coincides with the peak in the differential scanning calorimetric curve at 506°C. The assumption that the types of thermal destructions are different in different temperature ranges disagrees with the two ...
... to carbon(IV) oxide, i.e., to a deep oxidation with the maximum thermal effect at 502°C, nearly coincides with the peak in the differential scanning calorimetric curve at 506°C. The assumption that the types of thermal destructions are different in different temperature ranges disagrees with the two ...
Conduction electrons
... • Intermediate resistivity => “semiconductor” – conductivity lies between that of conductors and insulators – generally crystalline in structure for IC devices • In recent years, however, non-crystalline semiconductors have become commercially very important ...
... • Intermediate resistivity => “semiconductor” – conductivity lies between that of conductors and insulators – generally crystalline in structure for IC devices • In recent years, however, non-crystalline semiconductors have become commercially very important ...
Chapter 18 - Sarah Mahajan Study Guides
... Rate is a measure of speed of any change that occurs within an interval of time In chemistry, the reaction rate (rate of a chemical change) = amount of reactant per unit time o For example: 0.2 mol/1 month Collision theory- atoms, molecules, and ions can react to form products when they collide with ...
... Rate is a measure of speed of any change that occurs within an interval of time In chemistry, the reaction rate (rate of a chemical change) = amount of reactant per unit time o For example: 0.2 mol/1 month Collision theory- atoms, molecules, and ions can react to form products when they collide with ...
Chapter_Superconductivity
... of the lattice through electrostatic coulomb force, some electron momentum get transferred. As a result, these ions set up elastic wave in the lattice due to distortion. If another electron happens to pass through this region then the interaction between two occurs which in its effect lowers the ene ...
... of the lattice through electrostatic coulomb force, some electron momentum get transferred. As a result, these ions set up elastic wave in the lattice due to distortion. If another electron happens to pass through this region then the interaction between two occurs which in its effect lowers the ene ...
Statistical Mechanics Basis of Macleod`s Formula
... the critical temperature, K behaves as T4. It follows that the change in K with respect to the temperature is small because of the fact that for a given liquid/vapor system the change in temperature is not very important compared to the initial temperature. Near the critical temperature, the surface ...
... the critical temperature, K behaves as T4. It follows that the change in K with respect to the temperature is small because of the fact that for a given liquid/vapor system the change in temperature is not very important compared to the initial temperature. Near the critical temperature, the surface ...
Basic Concepts of the Gas Phase
... Gases appear to have no structure, size or shape. Different gases are always completely miscible and fill all space at their disposal, in contrast to liquids or solids, in which cohesive forces and surface tension restrain the thermal movement of molecules. Under the same conditions of temperature a ...
... Gases appear to have no structure, size or shape. Different gases are always completely miscible and fill all space at their disposal, in contrast to liquids or solids, in which cohesive forces and surface tension restrain the thermal movement of molecules. Under the same conditions of temperature a ...
Chapter 6 - Department of Chemical Engineering
... causing the vaporization of a liquid are derived from the kinetic energy of translation of its molecules. An increase in kinetic energy of molecular translation increases the rate of vaporization and vapor pressure; hence fewer particles tend to condense. The nature (or structure) of the liquid is t ...
... causing the vaporization of a liquid are derived from the kinetic energy of translation of its molecules. An increase in kinetic energy of molecular translation increases the rate of vaporization and vapor pressure; hence fewer particles tend to condense. The nature (or structure) of the liquid is t ...
Sound Wave Speed
... From the figure it is seen that Cv is equal to 3R at high temperatures regardless of the substance. This fact is known as Dulong-Petit law. This law states that specific heat of a given number of atoms of any solid is independent of temperature and is the same for all materials! ...
... From the figure it is seen that Cv is equal to 3R at high temperatures regardless of the substance. This fact is known as Dulong-Petit law. This law states that specific heat of a given number of atoms of any solid is independent of temperature and is the same for all materials! ...
The Science and Engineering of Materials, 4th ed Donald R
... turbine engines to operate more efficiently at higher temperatures. (Courtesy of Certech, Inc.) ...
... turbine engines to operate more efficiently at higher temperatures. (Courtesy of Certech, Inc.) ...
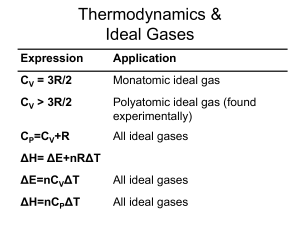
Thermodynamics
... any phase change energy) that has been dispersed in a system at a specific temperature. System with highest entropy has the greatest dispersal of energy For any spontaneous process, ΔSuniverse > 0 Units = J/K ...
... any phase change energy) that has been dispersed in a system at a specific temperature. System with highest entropy has the greatest dispersal of energy For any spontaneous process, ΔSuniverse > 0 Units = J/K ...
Name _____Mr. Perfect________________________________ Date __F 14_______ n l of
... 9. The first ionization energies of As and Se are 0.947 MJ/mol for As and 0.941 MJ/mol for Se. Explain this result using orbital diagrams. (5 pts) As [Ar] [↑↓] [↑↓][↑↓][↑↓][↑↓][↑↓] [↑ ][ ↑ ][↑ ] 4s 3d 4p Se [Ar] [↑↓] [↑↓][↑↓][↑↓][↑↓][↑↓] [↑↓][↑ ][↑ ] 4s 3d 4p The periodic trend would predict Se to h ...
... 9. The first ionization energies of As and Se are 0.947 MJ/mol for As and 0.941 MJ/mol for Se. Explain this result using orbital diagrams. (5 pts) As [Ar] [↑↓] [↑↓][↑↓][↑↓][↑↓][↑↓] [↑ ][ ↑ ][↑ ] 4s 3d 4p Se [Ar] [↑↓] [↑↓][↑↓][↑↓][↑↓][↑↓] [↑↓][↑ ][↑ ] 4s 3d 4p The periodic trend would predict Se to h ...
challenges in detecting crystalline phase in an amorphous
... characteristics of the APl are altered. One way to achieve the alteration is to stabilize the API in an amorphous phase. The higher energetic state of an amorphous phase often leads to significantly higher solubility, thus improving the bioavailability of drug molecule. Poorly soluble crystalline dr ...
... characteristics of the APl are altered. One way to achieve the alteration is to stabilize the API in an amorphous phase. The higher energetic state of an amorphous phase often leads to significantly higher solubility, thus improving the bioavailability of drug molecule. Poorly soluble crystalline dr ...
Glass transition
The glass–liquid transition or glass transition for short is the reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle state into a molten or rubber-like state. An amorphous solid that exhibits a glass transition is called a glass. Supercooling a viscous liquid into the glass state is called vitrification, from the Latin vitreum, ""glass"" via French vitrifier.Despite the massive change in the physical properties of a material through its glass transition, the transition is not itself a phase transition of any kind; rather it is a laboratory phenomenon extending over a range of temperature and defined by one of several conventions. Such conventions include a constant cooling rate (20 K/min) and a viscosity threshold of 1012 Pa·s, among others. Upon cooling or heating through this glass-transition range, the material also exhibits a smooth step in the thermal-expansion coefficient and in the specific heat, with the location of these effects again being dependent on the history of the material. However, the question of whether some phase transition underlies the glass transition is a matter of continuing research.The glass-transition temperature Tg is always lower than the melting temperature, Tm, of the crystalline state of the material, if one exists.