Telmisartan and Hydrochlorothiazide Tablets, USP 40 mg/12.5 mg
... 95-114 mmHg), 1031 of whom were treated with telmisartan. Following once daily administration of telmisartan, the magnitude of blood pressure reduction from baseline after placebo subtraction was approximately (SBP/DBP) 6-8/6 mmHg for 20 mg, 9-13/6-8 mmHg for 40 mg, and 12-13/7-8 mmHg for 80 mg. Lar ...
... 95-114 mmHg), 1031 of whom were treated with telmisartan. Following once daily administration of telmisartan, the magnitude of blood pressure reduction from baseline after placebo subtraction was approximately (SBP/DBP) 6-8/6 mmHg for 20 mg, 9-13/6-8 mmHg for 40 mg, and 12-13/7-8 mmHg for 80 mg. Lar ...
CONCERTA Extended-Release Tablets
... Metabolism: In humans, methylphenidate is metabolised primarily by de-esterification to -phenylpiperidine acetic acid (PPAA) which has little or no pharmacologic activity. In adults the metabolism of CONCERTA once daily, as evaluated by metabolism to PPAA, is similar to that of methylphenidate thre ...
... Metabolism: In humans, methylphenidate is metabolised primarily by de-esterification to -phenylpiperidine acetic acid (PPAA) which has little or no pharmacologic activity. In adults the metabolism of CONCERTA once daily, as evaluated by metabolism to PPAA, is similar to that of methylphenidate thre ...
QA248_4Pyridostigmine_neostigmine_FINAL
... The dose of pyridostigmine can vary from 30 - 120mg every 3-4 hours, with between 5-20 tablets taken daily.(2) The total daily dose of parenteral neostigmine can vary between 5-20mg, with 1-2.5mg given by IM or SC injection every 2-4 hours.(3) Oral neostigmine doses are usually 15 – 30mg at regular ...
... The dose of pyridostigmine can vary from 30 - 120mg every 3-4 hours, with between 5-20 tablets taken daily.(2) The total daily dose of parenteral neostigmine can vary between 5-20mg, with 1-2.5mg given by IM or SC injection every 2-4 hours.(3) Oral neostigmine doses are usually 15 – 30mg at regular ...
VIMOVO Prescribing Information
... Patients with a prior history of peptic ulcer disease and/or GI bleeding who used NSAIDs had a greater than 10-fold increased risk for developing a GI bleed compared to patients without these risk factors. Other factors that increase the risk for GI bleeding in patients treated with NSAIDs include l ...
... Patients with a prior history of peptic ulcer disease and/or GI bleeding who used NSAIDs had a greater than 10-fold increased risk for developing a GI bleed compared to patients without these risk factors. Other factors that increase the risk for GI bleeding in patients treated with NSAIDs include l ...
The new england journal of medicine
... clopidogrel, prasugrel, another thienopyridine prodrug, has a more consistent and pronounced inhibitory effect on platelets,5,6 resulting in a lower risk of myocardial infarction and stent thrombosis, but is associated with a higher risk of major bleeding in patients with an acute coronary syndrome ...
... clopidogrel, prasugrel, another thienopyridine prodrug, has a more consistent and pronounced inhibitory effect on platelets,5,6 resulting in a lower risk of myocardial infarction and stent thrombosis, but is associated with a higher risk of major bleeding in patients with an acute coronary syndrome ...
Overview of pleiotropic effects of platelet P2Y12receptor inhibitors
... which plays a pivotal role in platelet activation and aggregation. Stimulation of platelet P2Y12 receptor by ADP leads to a complex cascade of actions, which can result in arterial atherothrombosis, an important cause of adverse cardiovascular events. That is why the P2Y12 receptor has become an imp ...
... which plays a pivotal role in platelet activation and aggregation. Stimulation of platelet P2Y12 receptor by ADP leads to a complex cascade of actions, which can result in arterial atherothrombosis, an important cause of adverse cardiovascular events. That is why the P2Y12 receptor has become an imp ...
bicalutamide-ga - Actavis think smart medicine
... Bicalutamide is unlikely to impair the ability of patients to drive and operate machinery. Carcinogenesis, mutagenesis, impairment of fertility Carcinogenicity/ mutagenicity: Bicalutamide was inactive in in vitro tests for gene mutation and in in vitro and in vivo tests for clastogenicity. Two year ...
... Bicalutamide is unlikely to impair the ability of patients to drive and operate machinery. Carcinogenesis, mutagenesis, impairment of fertility Carcinogenicity/ mutagenicity: Bicalutamide was inactive in in vitro tests for gene mutation and in in vitro and in vivo tests for clastogenicity. Two year ...
Salair Inhaler
... Correct operation of the Salair Inhaler is essential for successful therapy. Prior to using the Salair Inhaler for the first time, remove the plastic dust cap from the mouthpiece of the inhaler, shake the inhaler and depress the canister twice into the air to prime the inhaler. If the inhaler has n ...
... Correct operation of the Salair Inhaler is essential for successful therapy. Prior to using the Salair Inhaler for the first time, remove the plastic dust cap from the mouthpiece of the inhaler, shake the inhaler and depress the canister twice into the air to prime the inhaler. If the inhaler has n ...
Zantac™ Syrup Zantac™ Tablets
... Gastro-oesophageal reflux disease:- Acute reflux oesophagitis:In reflux oesophagitis 150 mg twice daily or 300 mg nocte is administered for up to a period of 8, or if necessary, 12 weeks. In patients with moderate to severe oesophagitis, the dosage of ranitidine may be increased to 150 mg four times ...
... Gastro-oesophageal reflux disease:- Acute reflux oesophagitis:In reflux oesophagitis 150 mg twice daily or 300 mg nocte is administered for up to a period of 8, or if necessary, 12 weeks. In patients with moderate to severe oesophagitis, the dosage of ranitidine may be increased to 150 mg four times ...
Piracetam Nootropil - The Filipino Doctor
... The solution for injection will be administered intravenously over several minutes. The solution for infusion will be administered continuously at the recommended daily dose over a 24hour period. Recommended daily doses are provided below by indication. Symptomatic treatment of psycho-organic syndro ...
... The solution for injection will be administered intravenously over several minutes. The solution for infusion will be administered continuously at the recommended daily dose over a 24hour period. Recommended daily doses are provided below by indication. Symptomatic treatment of psycho-organic syndro ...
Nepafenac - Therapeutic Goods Administration
... performance (particle size, polymorphism and uniformity/homogeneity of dose) were identified and analysed appropriately. Compatibility of the drug substance and the excipients in the proposed container system has been evaluated. A stability study conducted on two lots of 0.3% nepafenac eye drops sus ...
... performance (particle size, polymorphism and uniformity/homogeneity of dose) were identified and analysed appropriately. Compatibility of the drug substance and the excipients in the proposed container system has been evaluated. A stability study conducted on two lots of 0.3% nepafenac eye drops sus ...
Isentress - Merck.com
... hypersensitivity reaction and toxic epidermal necrolysis. Immediately discontinue treatment with ISENTRESS or ISENTRESS HD and other suspect agents if severe hypersensitivity, severe rash, or rash with systemic symptoms or liver aminotransferase elevations develops and monitor clinical status, inclu ...
... hypersensitivity reaction and toxic epidermal necrolysis. Immediately discontinue treatment with ISENTRESS or ISENTRESS HD and other suspect agents if severe hypersensitivity, severe rash, or rash with systemic symptoms or liver aminotransferase elevations develops and monitor clinical status, inclu ...
TAMIFLU Product Monograph
... indicate a lower exposure to the active metabolite in pregnant women compared to non-pregnant women; however, this predicted exposure is expected to have clinical benefits and there are insufficient pharmacokinetic and safety data to recommend a dose adjustment in pregnant women who are justified to ...
... indicate a lower exposure to the active metabolite in pregnant women compared to non-pregnant women; however, this predicted exposure is expected to have clinical benefits and there are insufficient pharmacokinetic and safety data to recommend a dose adjustment in pregnant women who are justified to ...
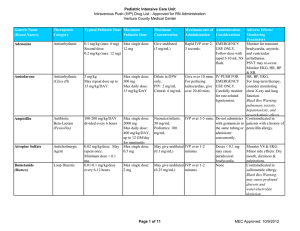
Pediatric Intensive Care Unit Intravenous Push (IVP) Drug List
... with active or recent bleeds, or taking aspirin or other NSAIDs. Black Box Warning: Increased risk of bleeding, nephrotoxicity, and cardiovascular thrombotic events. Medication is usually placed inline, but may be pushed. Use immediately after reconstitution. ...
... with active or recent bleeds, or taking aspirin or other NSAIDs. Black Box Warning: Increased risk of bleeding, nephrotoxicity, and cardiovascular thrombotic events. Medication is usually placed inline, but may be pushed. Use immediately after reconstitution. ...
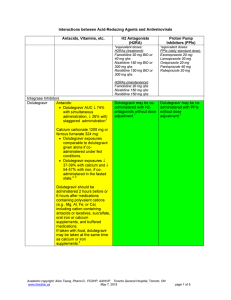
Interactions between Acid-Reducing Agents and Antiretrovirals
... Ranitidine 150 mg BID or 300 mg qhs ...
... Ranitidine 150 mg BID or 300 mg qhs ...
Basics Pharmacology Review - Dr. Roland Halil
... amount of time in preparation for LMCC • Apply knowledge of pharmacology to rationalize the application of therapeutics • List the four steps of rational prescribing • Understand the pharmacological classes, generic examples and mechanisms of action of important tools in the practice of medicine. • ...
... amount of time in preparation for LMCC • Apply knowledge of pharmacology to rationalize the application of therapeutics • List the four steps of rational prescribing • Understand the pharmacological classes, generic examples and mechanisms of action of important tools in the practice of medicine. • ...
Tafluprost/Timolol - Therapeutic Goods Administration
... darkening of the eyelid skin and increased iris pigmentation which are related to tafluprost therapy. Some of these changes may be permanent, and may lead to differences in appearance between the eyes when only one eye is treated. The change in iris pigmentation occurs slowly and may not be noticeab ...
... darkening of the eyelid skin and increased iris pigmentation which are related to tafluprost therapy. Some of these changes may be permanent, and may lead to differences in appearance between the eyes when only one eye is treated. The change in iris pigmentation occurs slowly and may not be noticeab ...
digibind - GSK Australia
... Standard therapy for digitalis intoxication includes withdrawal of the drug and correction of factors that may contribute to toxicity, such as electrolyte disturbances, hypoxia, acid-base disturbances and agents such as catecholamines. Also, treatment of arrhythmias may include judicious potassium s ...
... Standard therapy for digitalis intoxication includes withdrawal of the drug and correction of factors that may contribute to toxicity, such as electrolyte disturbances, hypoxia, acid-base disturbances and agents such as catecholamines. Also, treatment of arrhythmias may include judicious potassium s ...
TAMIFLU Product Monograph
... indicate a lower exposure to the active metabolite in pregnant women compared to non-pregnant women; however, this predicted exposure is expected to have clinical benefits and there are insufficient pharmacokinetic and safety data to recommend a dose adjustment in pregnant women who are justified to ...
... indicate a lower exposure to the active metabolite in pregnant women compared to non-pregnant women; however, this predicted exposure is expected to have clinical benefits and there are insufficient pharmacokinetic and safety data to recommend a dose adjustment in pregnant women who are justified to ...
Inhaled Corticosteroids - University of Michigan
... Oral corticosteroid use associated with increased risk of cataracts after only 1 year of cumulative treatment (compared to 3 years for ICS) Based on 1.75% baseline incidence of cataract extraction in study population, prolonged use of ICS will give rise to 361 additional cases of cataract extrac ...
... Oral corticosteroid use associated with increased risk of cataracts after only 1 year of cumulative treatment (compared to 3 years for ICS) Based on 1.75% baseline incidence of cataract extraction in study population, prolonged use of ICS will give rise to 361 additional cases of cataract extrac ...
Axiago gastro-resistant tablet ENG
... elimination half-life(t1/2) but no significant increase in peak plasma levels of cisapride. The slightly prolonged QTc interval observed after administration of cisapride alone, was not further prolonged when cisapride was given in combination with esomeprazole (see also section 4.4). Esomeprazole h ...
... elimination half-life(t1/2) but no significant increase in peak plasma levels of cisapride. The slightly prolonged QTc interval observed after administration of cisapride alone, was not further prolonged when cisapride was given in combination with esomeprazole (see also section 4.4). Esomeprazole h ...
Effects of Antidepressants on Inhibitory Avoidance in
... (Monleón et al., 2008). These studies provide several valuable insights into the effects of antidepressants on memory: ...
... (Monleón et al., 2008). These studies provide several valuable insights into the effects of antidepressants on memory: ...
SOLU-MEDROL Sterile Powder
... administered methylprednisolone sodium succinate compared to placebo (1.18 relative risk). A causal association with methylprednisolone sodium succinate treatment has not been established.42,43 Some studies do not establish the efficacy of methylprednisolone sodium succinate in septic shock, and sug ...
... administered methylprednisolone sodium succinate compared to placebo (1.18 relative risk). A causal association with methylprednisolone sodium succinate treatment has not been established.42,43 Some studies do not establish the efficacy of methylprednisolone sodium succinate in septic shock, and sug ...
NEXIUM ® I.V. - PI
... • Adults: Dose is either 20 mg or 40 mg NEXIUM given once daily by intravenous injection (no less than 3 minutes) or intravenous infusion (10 minutes to 30 minutes). • Pediatric: Give the following doses once daily as an intravenous infusion over 10 minutes to 30 minutes. (2.1): • 1 year to 17 ...
... • Adults: Dose is either 20 mg or 40 mg NEXIUM given once daily by intravenous injection (no less than 3 minutes) or intravenous infusion (10 minutes to 30 minutes). • Pediatric: Give the following doses once daily as an intravenous infusion over 10 minutes to 30 minutes. (2.1): • 1 year to 17 ...
Nitazoxanide (Alinia) - Texas Medicaid/CHIP Vendor Drug
... Nitazoxanide is rapidly metabolized to tizoxanide following oral administration. Because tizoxanide is highly bound to plasma proteins (>99.9%), caution should be exercised when dosing nitazoxanide concurrently with other drugs highly protein bound possessing narrow therapeutic indices as competitio ...
... Nitazoxanide is rapidly metabolized to tizoxanide following oral administration. Because tizoxanide is highly bound to plasma proteins (>99.9%), caution should be exercised when dosing nitazoxanide concurrently with other drugs highly protein bound possessing narrow therapeutic indices as competitio ...
Dydrogesterone

Dydrogesterone (INN, USAN, BAN), is also chemically known as 9β,10α-pregna-4,6-diene-3,20-dione. Dydrogesterone (6-dehydro-retroprogesterone) is a hormonally active, non-androgenic steroid that was developed in the 1950s.Dydrogesterone has selective progestational activity and does not inhibit ovulation. The greater rigidity of dydrogesterone also positively affects its selectivity, while natural progesterone is less selective, existing in different conformations that more easily bind to different receptors. As a consequence of its better bioavailability and the progestational activity of its main metabolites (20-, 21- and 16-hydroxy derivatives), the equivalent dose of dydrogesterone is 10–20 times lower than that of oral micronized progesterone.Dydrogesterone is used as an effective, orally active progestogen for gynaecological conditions related to a wide variety of progesterone deficiencies in pregnant women. The molecular structure and pharmacological effects are somewhat similar to endogenous progesterone, although in smaller amounts it is found to be orally active. Its freedom from hormonal effects like those related to corticoid, androgenic, estrogenic, anabolic, and other effects gives dydrogesterone an advantage over other synthesized progestogens.Dydrogesterone when used therapeutically is closely related to its physiological action on the neuro-endocrine control of ovarian function, as well as on the endometrium. This is an indication in all cases of endogeneous progesterone deficiency - relative or absolute. The molecule was licensed for use in several indications, including threatened or recurrent miscarriage, dysfunctional bleeding, infertility due to luteal insufficiency, dysmenorrhea, endometriosis, secondary amenorrhoea, irregular cycles, pre-menstrual syndrome and also as a hormone replacement therapy.Dydrogesterone has proven effective in the following conditions associated with progesterone deficiency: Infertility due to luteal insufficiency Threatened miscarriage Habitual or recurrent miscarriage. Menstrual disorders Premenstrual syndrome Endometriosis Dydrogesterone has also been registered as hormone replacement therapy (HRT) to counter-check the negative effects of unopposed estrogen on the endometrium in women with an intact uterus. Dydrogesterone is relatively safe and well tolerated, and does not exhibit the androgenic side effects that are common with some other progestins, like medroxyprogesterone acetate.