název projektu
... •A perpetual motion machine of the first kind produces work without the input of energy. It thus violates the first law of thermodynamics: the law of conservation of energy. •A perpetual motion machine of the second kind is a machine which spontaneously converts thermal energy into mechanical work. ...
... •A perpetual motion machine of the first kind produces work without the input of energy. It thus violates the first law of thermodynamics: the law of conservation of energy. •A perpetual motion machine of the second kind is a machine which spontaneously converts thermal energy into mechanical work. ...
entropy - Helios
... Gas diffuses throughout a room because the probability of a configuration where all of the molecules bunch up is low ...
... Gas diffuses throughout a room because the probability of a configuration where all of the molecules bunch up is low ...
6. Absorption of Heat
... HRW 75E (5th ed.). Gas within a chamber passes through the cycle shown in Fig. 19-37. Determine the net heat added to the system during process CA if the heat QAB added during process AB is 20.0 J, no heat is transferred during process BC, and the net work dome during the cycle is 15.0 J. Since the ...
... HRW 75E (5th ed.). Gas within a chamber passes through the cycle shown in Fig. 19-37. Determine the net heat added to the system during process CA if the heat QAB added during process AB is 20.0 J, no heat is transferred during process BC, and the net work dome during the cycle is 15.0 J. Since the ...
1st law of Thermodynamics Worksheet
... Thermodynamics is the study of processes in which energy is transferred as heat and as work. 2. What is internal energy? The sum total of all the energy of the molecules of the system. 3. State the first law of thermodynamics. The change of internal energy of a closed system will be equal to the heat ...
... Thermodynamics is the study of processes in which energy is transferred as heat and as work. 2. What is internal energy? The sum total of all the energy of the molecules of the system. 3. State the first law of thermodynamics. The change of internal energy of a closed system will be equal to the heat ...
Work Done - akamdiplomaphysics
... • carry out the process rapidly so that the heat does not have the time to leave the system ...
... • carry out the process rapidly so that the heat does not have the time to leave the system ...
Chapter 12
... A state variable related to the Second Law of Thermodynamics, the entropy Let Qr be the energy absorbed or expelled during a reversible, constant temperature process between two equilibrium states. Then the change in entropy during any constant temperature process connecting the two equilibrium stat ...
... A state variable related to the Second Law of Thermodynamics, the entropy Let Qr be the energy absorbed or expelled during a reversible, constant temperature process between two equilibrium states. Then the change in entropy during any constant temperature process connecting the two equilibrium stat ...
Chapter2 The First Law of Thermodynamics
... denote as Q ----------kJ heat transferred per unit mass of a system is denoted as q----------kJ/kg Q ...
... denote as Q ----------kJ heat transferred per unit mass of a system is denoted as q----------kJ/kg Q ...
here
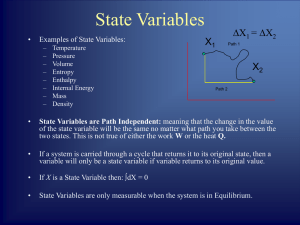
... If A and B are each in thermal equilibrium with a third body C, then A and B are in thermal equilibrium. Thermal equilibrium means that two bodies are in states such that if they are connected, then their condition will not change. ...
... If A and B are each in thermal equilibrium with a third body C, then A and B are in thermal equilibrium. Thermal equilibrium means that two bodies are in states such that if they are connected, then their condition will not change. ...
1 Lecture: 2 Thermodynamic equilibrium 1
... (8)). We adopt the convention that W > 0 if the energy of the system increases. During a process the internal energy of the system increases if the process that caused the change can be produced by lowering a weight somewhere in the surrounding of the system and using this mechanical work to cause t ...
... (8)). We adopt the convention that W > 0 if the energy of the system increases. During a process the internal energy of the system increases if the process that caused the change can be produced by lowering a weight somewhere in the surrounding of the system and using this mechanical work to cause t ...
3.3 and 3.4 Non Flow Energy
... Hussein) or the water in the kettle). When dealing with a non-flow situation, then the system will be of fixed mass - no matter crosses the boundary - so it is useful to define a control mass. If we are considering a flow situation, then a control volume through which the fluid flows is more useful. ...
... Hussein) or the water in the kettle). When dealing with a non-flow situation, then the system will be of fixed mass - no matter crosses the boundary - so it is useful to define a control mass. If we are considering a flow situation, then a control volume through which the fluid flows is more useful. ...
Thermodynamics
... In other words: All processes are reversible (Reversible means that it is possible to return system and surroundings to the initial states) ...
... In other words: All processes are reversible (Reversible means that it is possible to return system and surroundings to the initial states) ...
Homework 3
... With the above definition, when we add heat to the system, dQ is positive. Conversely dQ is negative when heat is removed from the system. When we compress a system, we expend energy in act of doing so and this energy gets stored in the system or gets converted to heat or both. Examples would be com ...
... With the above definition, when we add heat to the system, dQ is positive. Conversely dQ is negative when heat is removed from the system. When we compress a system, we expend energy in act of doing so and this energy gets stored in the system or gets converted to heat or both. Examples would be com ...
Lect1.LawsofThr
... dE = dQ-dW d indicates inexact differential - depends on the integration path In a cycle, E = 0 net Q in = net W out Work and heat are not state functions Energy is a state function dW = Fdx can be PdV, -dl, -it ...
... dE = dQ-dW d indicates inexact differential - depends on the integration path In a cycle, E = 0 net Q in = net W out Work and heat are not state functions Energy is a state function dW = Fdx can be PdV, -dl, -it ...