File - El Paso High School
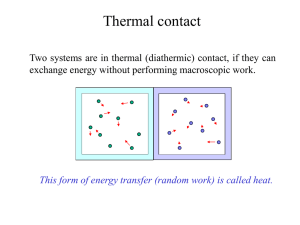
... - An example of an isothermal system is a gas confined by a piston in a cylinder where the gas is not heated or cooled, but the piston is slowly moved so that the gas expands or is compressed. The temperature is maintained at a constant value by putting the system in contact with a constant-temperat ...
... - An example of an isothermal system is a gas confined by a piston in a cylinder where the gas is not heated or cooled, but the piston is slowly moved so that the gas expands or is compressed. The temperature is maintained at a constant value by putting the system in contact with a constant-temperat ...
Thermodynamics Temperature Scales Thermal Expansion and Stress
... may happen through heat transfer or through mechanical work First law is a statement of conservation of energy Change in internal energy of system equals the difference between the heat added to the system and the work done by the system ΔU = Q − W ...
... may happen through heat transfer or through mechanical work First law is a statement of conservation of energy Change in internal energy of system equals the difference between the heat added to the system and the work done by the system ΔU = Q − W ...
States of matter - Tennessee State University
... Internal energy of an ideal gas The internal energy of an ideal gas results from the translational kinetic energy, rotational kinetic energy, and vibrational energy of the molecules constituting the system. According to the kinetic theory of gases, the internal energy of an ideal gas is a function ...
... Internal energy of an ideal gas The internal energy of an ideal gas results from the translational kinetic energy, rotational kinetic energy, and vibrational energy of the molecules constituting the system. According to the kinetic theory of gases, the internal energy of an ideal gas is a function ...
U3MEA02 Basic Engineering Thermodynamics
... • In his ideal model, the heat of caloric converted into work could be reinstated by reversing the motion of the cycle, a concept subsequently known as thermodynamic reversibility. Carnot however further postulated that some caloric is lost, not being converted to mechanical work. Hence no real heat ...
... • In his ideal model, the heat of caloric converted into work could be reinstated by reversing the motion of the cycle, a concept subsequently known as thermodynamic reversibility. Carnot however further postulated that some caloric is lost, not being converted to mechanical work. Hence no real heat ...
Internal Energy and the State of a System A system (e.g., a steam
... Internal Energy and the State of a System The state of a system refers to the quintessential properties fully define a system’s characteristics. Internal energy U is part of the description of the state of a system – the energy it has by just sitting there, not macroscopically moving. Its main const ...
... Internal Energy and the State of a System The state of a system refers to the quintessential properties fully define a system’s characteristics. Internal energy U is part of the description of the state of a system – the energy it has by just sitting there, not macroscopically moving. Its main const ...
Thermodynamics
... Energy. When a system gains heat, the internal energy of the system increases. Q is positive when a system gains heat and negative when a system loses heat. Internal energy of a system can decrease if the system does work on its surroundings. Work is positive when it is done by the system and negati ...
... Energy. When a system gains heat, the internal energy of the system increases. Q is positive when a system gains heat and negative when a system loses heat. Internal energy of a system can decrease if the system does work on its surroundings. Work is positive when it is done by the system and negati ...
Tutorial II (thermodynamics)
... or false giving your thermodynamic reasoning. If the statement is false you may either state which law or laws of thermodynamics it violates or provide a physical counter example or any other plausible physical reason. Finally, correct the false statement with a clarifying phrase that makes the stat ...
... or false giving your thermodynamic reasoning. If the statement is false you may either state which law or laws of thermodynamics it violates or provide a physical counter example or any other plausible physical reason. Finally, correct the false statement with a clarifying phrase that makes the stat ...
PV Diagrams
... 2. The other way to have an adiabatic process (this is actually called a “near adiabatic process”) is to have it happen very quickly. The process happens so fast that there is no time for heat to be transferred. The combustion of gasoline in an engine is considered to be adiabatic ...
... 2. The other way to have an adiabatic process (this is actually called a “near adiabatic process”) is to have it happen very quickly. The process happens so fast that there is no time for heat to be transferred. The combustion of gasoline in an engine is considered to be adiabatic ...
Vocabulary - cloudfront.net
... he can hear the click of the train going over spaces between the rails. Six months later, on a 30.0 ºC summer day, the rails are pushed tightly together and he hears no click. IF the rails are 5.00 m long, how large a gap is left between the rails on the cold winter day? (αsteel = 12 x 10-6 ºC-1). ...
... he can hear the click of the train going over spaces between the rails. Six months later, on a 30.0 ºC summer day, the rails are pushed tightly together and he hears no click. IF the rails are 5.00 m long, how large a gap is left between the rails on the cold winter day? (αsteel = 12 x 10-6 ºC-1). ...
Chap 7 - College of Science | Oregon State University
... In practice, the efficiency is always less than ideal because of friction (mostly.) Gasoline engine actual efficiency = 10-15% Refrigerators, Air Conditioners, Heat Pumps are “heat engines in reverse”. Instead of moving thermal energy around to output work (motion), we put in the work (a motor drive ...
... In practice, the efficiency is always less than ideal because of friction (mostly.) Gasoline engine actual efficiency = 10-15% Refrigerators, Air Conditioners, Heat Pumps are “heat engines in reverse”. Instead of moving thermal energy around to output work (motion), we put in the work (a motor drive ...
FE Review Common Pitfalls in Thermodynamics
... This result is correct only when the process is constant pressure, and P = P1 = P2. 8. Temperature—When the value of temperature is substituted into an equation such as the ideal equation of state, the temperature must be in absolute units. A temperature change in the ordinary units, celsius or fahr ...
... This result is correct only when the process is constant pressure, and P = P1 = P2. 8. Temperature—When the value of temperature is substituted into an equation such as the ideal equation of state, the temperature must be in absolute units. A temperature change in the ordinary units, celsius or fahr ...
Objectives Recognize that a system can absorb or release energy
... A situation in which pressure ________________________and volume remains constant is comparable to one in which a force does not displace a mass even as the force is increased. _____________ is not done in either situation. ...
... A situation in which pressure ________________________and volume remains constant is comparable to one in which a force does not displace a mass even as the force is increased. _____________ is not done in either situation. ...