Basic Concepts - Department of Chemistry
... – A chemical equilibrium is a reversible reaction that the forward reaction rate is equal to the reverse reaction rate. • Chemical equilibria are dynamic equilibria. – Molecules are continually reacting, even though the overall composition of the reaction mixture does not change. – Double arrow (⇋) ...
... – A chemical equilibrium is a reversible reaction that the forward reaction rate is equal to the reverse reaction rate. • Chemical equilibria are dynamic equilibria. – Molecules are continually reacting, even though the overall composition of the reaction mixture does not change. – Double arrow (⇋) ...
Basic Concepts
... – A chemical equilibrium is a reversible reaction that the forward reaction rate is equal to the reverse reaction rate. • Chemical equilibria are dynamic equilibria. – Molecules are continually reacting, even though the overall composition of the reaction mixture does not change. – Double arrow (⇋) ...
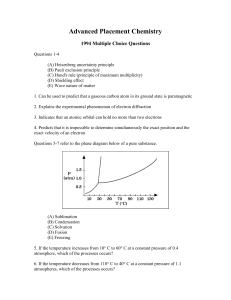
... – A chemical equilibrium is a reversible reaction that the forward reaction rate is equal to the reverse reaction rate. • Chemical equilibria are dynamic equilibria. – Molecules are continually reacting, even though the overall composition of the reaction mixture does not change. – Double arrow (⇋) ...
Solution FRQs Practice
... (c) Calculate the mole fraction of benzene in the solution described above. (d) The vapor pressure of pure benzene at 35C is 150. millimeters of Hg. Calculate the vapor pressure of benzene over the solution described above at 35C. ...
... (c) Calculate the mole fraction of benzene in the solution described above. (d) The vapor pressure of pure benzene at 35C is 150. millimeters of Hg. Calculate the vapor pressure of benzene over the solution described above at 35C. ...
Dr David`s Chemistry Revision Themes
... experience similar shielding. There is a regular contraction in overall atom size, from Na to Cl, as the nuclear charge increases and the electrons are drawn in towards the nucleus. 4. Explain why element C (in the table above) has a much higher melting point than krypton (they are next to one anoth ...
... experience similar shielding. There is a regular contraction in overall atom size, from Na to Cl, as the nuclear charge increases and the electrons are drawn in towards the nucleus. 4. Explain why element C (in the table above) has a much higher melting point than krypton (they are next to one anoth ...
Chapter 13: Water and the Lithosphere Preview
... the hydrated form, Si(OH)4. This form, silicic acid, exists as free molecules in very dilute solutions. As rain falls on feldspar, the sodium bicarbonate and silicic acid gradually leach away, leaving kaolinite behind. This is a very slow process, but the accumulation of kaolinite, and of other clay ...
... the hydrated form, Si(OH)4. This form, silicic acid, exists as free molecules in very dilute solutions. As rain falls on feldspar, the sodium bicarbonate and silicic acid gradually leach away, leaving kaolinite behind. This is a very slow process, but the accumulation of kaolinite, and of other clay ...
2nd Semester final review
... Kelvin has no negative numbers. The smallest Kelvin temperature is zero which is -273 oC ...
... Kelvin has no negative numbers. The smallest Kelvin temperature is zero which is -273 oC ...
Chapter 8 and 9 homework
... 24. The concentration of Cu2+ ions in the water (which also contains sulfate ions) discharged from a certain industrial plant is determined by adding excess sodium sulfide (Na2S) solution to 0.800 L of the water. The molecular equation is: Na2S(aq) + CuSO4(aq) →Na2SO4(aq) + CuS(s) Calculate the mola ...
... 24. The concentration of Cu2+ ions in the water (which also contains sulfate ions) discharged from a certain industrial plant is determined by adding excess sodium sulfide (Na2S) solution to 0.800 L of the water. The molecular equation is: Na2S(aq) + CuSO4(aq) →Na2SO4(aq) + CuS(s) Calculate the mola ...
AP Reactions - Georgetown ISD
... Keywords such as "excess" and "concentrated" of any solution may indicate complex ions. AgNO3 + HCl forms the white precipitate, AgCl. With excess, concentrated HCl, the complex ion, AgCl2-, forms and the solution clears. The odd complex ion, FeSCN2+, shows up once in a while simply because it is co ...
... Keywords such as "excess" and "concentrated" of any solution may indicate complex ions. AgNO3 + HCl forms the white precipitate, AgCl. With excess, concentrated HCl, the complex ion, AgCl2-, forms and the solution clears. The odd complex ion, FeSCN2+, shows up once in a while simply because it is co ...
Worksheet 8 Notes - Department of Chemistry | Oregon State
... the other product is 6027X because the mass number on both sides of the reaction arrow is 60 and the atomic number on both sides of the reaction arrow is 27. This corresponds to 6027Co. 60m27Co is in the excited state and will emit a gamma ray to become 6027Co. 7. A student isolates a sample of trit ...
... the other product is 6027X because the mass number on both sides of the reaction arrow is 60 and the atomic number on both sides of the reaction arrow is 27. This corresponds to 6027Co. 60m27Co is in the excited state and will emit a gamma ray to become 6027Co. 7. A student isolates a sample of trit ...
CHEM 102 FINAL EXAM WINTER 07-08
... 23. In a 1.2 M solution of HClO4, a strong acid, [H3O+] = _________, and [OH-] = _________. a. 1.0 × 10-7 M; 1.0 × 10-7 M b. 8.3 × 10-15 M; 1.2 M c. 1.2 M; 8.3 × 10-15 M d. 1.2 M; 1.2 M ANSWER: c 24. The value of the ionization constant for a weak acid HA is 4.2 × 10-7. What is the pH of a 0.35 M s ...
... 23. In a 1.2 M solution of HClO4, a strong acid, [H3O+] = _________, and [OH-] = _________. a. 1.0 × 10-7 M; 1.0 × 10-7 M b. 8.3 × 10-15 M; 1.2 M c. 1.2 M; 8.3 × 10-15 M d. 1.2 M; 1.2 M ANSWER: c 24. The value of the ionization constant for a weak acid HA is 4.2 × 10-7. What is the pH of a 0.35 M s ...
Honors Chemistry / SAT II
... 2488. The maximum numbers of electrons in the K, L, M, and N shells of any element are respectively (A) 1, 2, 8, 16 (D) 2, 8, 18, 32 (B) 1, 4, 9, 16 (E) 2, 6, 10, 14 (C) 2, 8, 16, 24 ...
... 2488. The maximum numbers of electrons in the K, L, M, and N shells of any element are respectively (A) 1, 2, 8, 16 (D) 2, 8, 18, 32 (B) 1, 4, 9, 16 (E) 2, 6, 10, 14 (C) 2, 8, 16, 24 ...
Experiment 1
... To become familiar with the operation of a pH meter and quantitative equilibrium constants. ...
... To become familiar with the operation of a pH meter and quantitative equilibrium constants. ...
Name: 1) At 1 atmosphere and 298 K, 1 mole of H O(l) molecules
... Careful observation reveals that these bubbles rise to the surface because CO2 gas is much less dense than water. However, not all of the CO2 gas rises to the surface; some of it dissolves in the water. The dissolved CO2 can react with water to form carbonic acid, H2 CO3 . ...
... Careful observation reveals that these bubbles rise to the surface because CO2 gas is much less dense than water. However, not all of the CO2 gas rises to the surface; some of it dissolves in the water. The dissolved CO2 can react with water to form carbonic acid, H2 CO3 . ...
Acid
An acid (from the Latin acidus/acēre meaning sour) is a chemical substance whose aqueous solutions are characterized by a sour taste, the ability to turn blue litmus red, and the ability to react with bases and certain metals (like calcium) to form salts. Aqueous solutions of acids have a pH of less than 7. Non-aqueous acids are usually formed when an anion (negative ion) reacts with one or more positively charged hydrogen cations. A lower pH means a higher acidity, and thus a higher concentration of positive hydrogen ions in the solution. Chemicals or substances having the property of an acid are said to be acidic.There are three common definitions for acids: the Arrhenius definition, the Brønsted-Lowry definition, and the Lewis definition. The Arrhenius definition defines acids as substances which increase the concentration of hydrogen ions (H+), or more accurately, hydronium ions (H3O+), when dissolved in water. The Brønsted-Lowry definition is an expansion: an acid is a substance which can act as a proton donor. By this definition, any compound which can easily be deprotonated can be considered an acid. Examples include alcohols and amines which contain O-H or N-H fragments. A Lewis acid is a substance that can accept a pair of electrons to form a covalent bond. Examples of Lewis acids include all metal cations, and electron-deficient molecules such as boron trifluoride and aluminium trichloride.Common examples of acids include hydrochloric acid (a solution of hydrogen chloride which is found in gastric acid in the stomach and activates digestive enzymes), acetic acid (vinegar is a dilute solution of this liquid), sulfuric acid (used in car batteries), and tartaric acid (a solid used in baking). As these examples show, acids can be solutions or pure substances, and can be derived from solids, liquids, or gases. Strong acids and some concentrated weak acids are corrosive, but there are exceptions such as carboranes and boric acid.