Chapters 14 and 15 Outline
... pH meter – is a device that determines the pH of a solution by measuring the voltage between the two electrodes that are in the solution. Titration – is a controlled addition and measurement of the amount of a solution of known concentration required to react completely with a measure of a solution ...
... pH meter – is a device that determines the pH of a solution by measuring the voltage between the two electrodes that are in the solution. Titration – is a controlled addition and measurement of the amount of a solution of known concentration required to react completely with a measure of a solution ...
Calculating a Ka Value from a Known pH - Chemwiki
... Ka, the acid ionization constant, is the equilibrium constant for chemical reactions involving weak acids in aqueous solution. The numerical value of Ka is used to predict the extent of acid dissociation. A large Ka value indicates a stronger acid (more of the acid dissociates) and small Ka val ...
... Ka, the acid ionization constant, is the equilibrium constant for chemical reactions involving weak acids in aqueous solution. The numerical value of Ka is used to predict the extent of acid dissociation. A large Ka value indicates a stronger acid (more of the acid dissociates) and small Ka val ...
7.2 Acids and Bases
... Acids Reacts with metals and carbonates Conducts electricity Turns blue litmus paper red Tastes sour pH < 7 Neutralizes bases ...
... Acids Reacts with metals and carbonates Conducts electricity Turns blue litmus paper red Tastes sour pH < 7 Neutralizes bases ...
8. Acids and bases
... • Conjugate acid-base pairs differs by just one proton. • A strong acid (HCl) has a weak conjugate base (Cl-) • A weak acid (H2O) has a strong conjugate base. ...
... • Conjugate acid-base pairs differs by just one proton. • A strong acid (HCl) has a weak conjugate base (Cl-) • A weak acid (H2O) has a strong conjugate base. ...
Give formulas of these acids, bases and salts boron silicide
... 1. A solution has a pH of 4 - what does this mean? It is acidic. It is neutral. It is alkaline. 2. Which of the statements below is correct? Bases are acids that dissolve in water. Bases are alkalis that dissolve in water. Alkalis are bases that dissolve in water. 3. A liquid has a pH of 7. What doe ...
... 1. A solution has a pH of 4 - what does this mean? It is acidic. It is neutral. It is alkaline. 2. Which of the statements below is correct? Bases are acids that dissolve in water. Bases are alkalis that dissolve in water. Alkalis are bases that dissolve in water. 3. A liquid has a pH of 7. What doe ...
Quiz 1
... 7. Which of the following is a correct statement concerning solution A with a pH of 11.5 compared to solution B with a pH of 10.0? Solution A… a. has a smaller [OH¯] than solution B b. has a larger number of [H+] than solution B c. is more basic than solution B d. is more acidic than solution B e. h ...
... 7. Which of the following is a correct statement concerning solution A with a pH of 11.5 compared to solution B with a pH of 10.0? Solution A… a. has a smaller [OH¯] than solution B b. has a larger number of [H+] than solution B c. is more basic than solution B d. is more acidic than solution B e. h ...
Acid and Bases 2
... hydroxide ions in solution. Example: HCl + NaOH NaCl + H2O **BASICALLY…acids start with H+ and bases end with OH-!!! ...
... hydroxide ions in solution. Example: HCl + NaOH NaCl + H2O **BASICALLY…acids start with H+ and bases end with OH-!!! ...
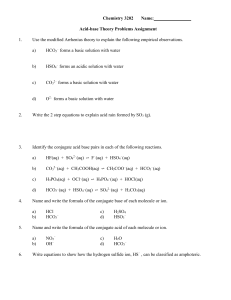
Chemistry 3202 Name: Acid-base Theory Problems Assignment 1
... Strong acids, such as perchloric acid, have been shown to react quantitatively with strong bases, such as sodium hydroxide. ...
... Strong acids, such as perchloric acid, have been shown to react quantitatively with strong bases, such as sodium hydroxide. ...
acids: bases - IDS-chem2-Rn-10
... BASE: •A base in chemistry is an aqueous substance that can accept hydronium ions. ...
... BASE: •A base in chemistry is an aqueous substance that can accept hydronium ions. ...
Answer on Question #44399 – Chemistry – Other HC2O4 − + HOH
... Answer According to the Brønsted–Lowry theory, an acid is a species able to lose, or "donate" a proton (H+) while a base is a species with the ability to gain, or "accept," a proton. The hydrogen oxalate ion can gain a proton acting as a base towards water, while the latter donates proton acting as ...
... Answer According to the Brønsted–Lowry theory, an acid is a species able to lose, or "donate" a proton (H+) while a base is a species with the ability to gain, or "accept," a proton. The hydrogen oxalate ion can gain a proton acting as a base towards water, while the latter donates proton acting as ...
Acid
An acid (from the Latin acidus/acēre meaning sour) is a chemical substance whose aqueous solutions are characterized by a sour taste, the ability to turn blue litmus red, and the ability to react with bases and certain metals (like calcium) to form salts. Aqueous solutions of acids have a pH of less than 7. Non-aqueous acids are usually formed when an anion (negative ion) reacts with one or more positively charged hydrogen cations. A lower pH means a higher acidity, and thus a higher concentration of positive hydrogen ions in the solution. Chemicals or substances having the property of an acid are said to be acidic.There are three common definitions for acids: the Arrhenius definition, the Brønsted-Lowry definition, and the Lewis definition. The Arrhenius definition defines acids as substances which increase the concentration of hydrogen ions (H+), or more accurately, hydronium ions (H3O+), when dissolved in water. The Brønsted-Lowry definition is an expansion: an acid is a substance which can act as a proton donor. By this definition, any compound which can easily be deprotonated can be considered an acid. Examples include alcohols and amines which contain O-H or N-H fragments. A Lewis acid is a substance that can accept a pair of electrons to form a covalent bond. Examples of Lewis acids include all metal cations, and electron-deficient molecules such as boron trifluoride and aluminium trichloride.Common examples of acids include hydrochloric acid (a solution of hydrogen chloride which is found in gastric acid in the stomach and activates digestive enzymes), acetic acid (vinegar is a dilute solution of this liquid), sulfuric acid (used in car batteries), and tartaric acid (a solid used in baking). As these examples show, acids can be solutions or pure substances, and can be derived from solids, liquids, or gases. Strong acids and some concentrated weak acids are corrosive, but there are exceptions such as carboranes and boric acid.