Unit 6 Review Answers
... (d) Students may say that H3PO4(aq) is the stronger acid because it is higher in the list of relative strengths of acids and bases. Some students may suggest that H3PO4(aq) is a stronger acid because its Ka value is larger than that of H2PO4−(aq), which is a “stronger” answer. 50. Water could have a ...
... (d) Students may say that H3PO4(aq) is the stronger acid because it is higher in the list of relative strengths of acids and bases. Some students may suggest that H3PO4(aq) is a stronger acid because its Ka value is larger than that of H2PO4−(aq), which is a “stronger” answer. 50. Water could have a ...
Chemistry Spell check on
... Reference may be made to the Chemistry Higher and Advanced Higher Data Booklet. SECTION A—Questions 1–40 (40 marks) Instructions for completion of Section A are given on page two. For this section of the examination you must use an HB pencil. SECTION B (60 marks) 1 All questions should be attempted ...
... Reference may be made to the Chemistry Higher and Advanced Higher Data Booklet. SECTION A—Questions 1–40 (40 marks) Instructions for completion of Section A are given on page two. For this section of the examination you must use an HB pencil. SECTION B (60 marks) 1 All questions should be attempted ...
Document
... Reference may be made to the Chemistry Higher and Advanced Higher Data Booklet. SECTION A—Questions 1–40 (40 marks) Instructions for completion of Section A are given on page two. For this section of the examination you must use an HB pencil. SECTION B (60 marks) 1 All questions should be attempted ...
... Reference may be made to the Chemistry Higher and Advanced Higher Data Booklet. SECTION A—Questions 1–40 (40 marks) Instructions for completion of Section A are given on page two. For this section of the examination you must use an HB pencil. SECTION B (60 marks) 1 All questions should be attempted ...
Questions 1-2
... For each of the following three reactions, write a balanced equation in part (1) and answer the question in part (2). In part (1), coefficients should be in terms of lowest whole numbers. Assume that solutions are aqueous unless otherwise indicated. Represent substances in solutions as ions if the s ...
... For each of the following three reactions, write a balanced equation in part (1) and answer the question in part (2). In part (1), coefficients should be in terms of lowest whole numbers. Assume that solutions are aqueous unless otherwise indicated. Represent substances in solutions as ions if the s ...
LEGGETT--AP CHEMISTRY * MINIMAL FINAL REVIEW
... C. Net energy change is zero D. Exothermic or endothermic depending on conditions. 17. How many sigma (σ) and pi(π) electron pairs are there in a carbon dioxide molecule? A. Two sigma, zero pi B. One sigma, one pi C. Two sigma, two pi D. Two sigma, one pi 18. Which of the following elements is most ...
... C. Net energy change is zero D. Exothermic or endothermic depending on conditions. 17. How many sigma (σ) and pi(π) electron pairs are there in a carbon dioxide molecule? A. Two sigma, zero pi B. One sigma, one pi C. Two sigma, two pi D. Two sigma, one pi 18. Which of the following elements is most ...
Document
... C) The element is located in period 2 and is an alkali metal. D) The element is located in period 2 and is an alkaline earth metal. 3. In the diagram below, the circles numbered 1 to 6 represent a characteristic shared by categories of elements in the periodic table. ...
... C) The element is located in period 2 and is an alkali metal. D) The element is located in period 2 and is an alkaline earth metal. 3. In the diagram below, the circles numbered 1 to 6 represent a characteristic shared by categories of elements in the periodic table. ...
Chapter 3: Calculations with Chemical Formulas
... The molecular equation for the process is: CaCO3(s) + 2HNO3(aq) Ca(NO3)2(aq) + H2O(l) + CO2(g) The corresponding net ionic equation is CaCO3(s) + 2H+(aq) Ca2+(aq) + H2O(l) + CO2(g) ...
... The molecular equation for the process is: CaCO3(s) + 2HNO3(aq) Ca(NO3)2(aq) + H2O(l) + CO2(g) The corresponding net ionic equation is CaCO3(s) + 2H+(aq) Ca2+(aq) + H2O(l) + CO2(g) ...
Review for Final Exam - Short Answer and Problems
... KP = 0.250 at 1100 K for the following equilibrium: 2 SO2 (g) + O2 (g) 2 SO3 (g) For this reaction K = ________________________, and for the following reaction: SO2 (g) + 1/2 O2 (g) SO3 (g) KP = __________________________ . ...
... KP = 0.250 at 1100 K for the following equilibrium: 2 SO2 (g) + O2 (g) 2 SO3 (g) For this reaction K = ________________________, and for the following reaction: SO2 (g) + 1/2 O2 (g) SO3 (g) KP = __________________________ . ...
Practice Writing AP Questions
... 11. A piece of lithium metal is dropped into a container of nitrogen gas. a. If the container was sealed quickly, what would you expect to happen to the pressure inside the container as the reaction proceeds? 12. Powdered magnesium oxide is added to a container of carbon dioxide gas. a. State the ox ...
... 11. A piece of lithium metal is dropped into a container of nitrogen gas. a. If the container was sealed quickly, what would you expect to happen to the pressure inside the container as the reaction proceeds? 12. Powdered magnesium oxide is added to a container of carbon dioxide gas. a. State the ox ...
10th CBSE {SA - 1} Revision Pack Booklet - 3
... 3. State Arrhenius concept of acids and bases. Select a strong acid and a weak base from amongst the following substances H2CO3, HNO3, NaOH, NH4OH. Sol: According to Arrhenius theory, acids are substances which provide H+ ions when dissolved in water, whereas bases are substances which when dissolve ...
... 3. State Arrhenius concept of acids and bases. Select a strong acid and a weak base from amongst the following substances H2CO3, HNO3, NaOH, NH4OH. Sol: According to Arrhenius theory, acids are substances which provide H+ ions when dissolved in water, whereas bases are substances which when dissolve ...
Chemistry 1B General Chemistry Exp 1 Spring 2017
... experiment in your own words. You will most likely need to consult your text book as well as your lab manual for additional information. Please cite any references (see below for more information on this). • Experimental Procedures: You will need to summarize the procedures for the experiment. You d ...
... experiment in your own words. You will most likely need to consult your text book as well as your lab manual for additional information. Please cite any references (see below for more information on this). • Experimental Procedures: You will need to summarize the procedures for the experiment. You d ...
Time
... If an equilibrium constant for a system has a value of 5.62 x 1049, would you expect to find mostly reactant or mostly product after the system has come to equilibrium? ...
... If an equilibrium constant for a system has a value of 5.62 x 1049, would you expect to find mostly reactant or mostly product after the system has come to equilibrium? ...
C H
... 1) Carbon atoms are not usually shown intersections, end of each line 2) Hydrogen atoms bonded to C are not shown. They are shown in cases when they are attached to heteroatom 3) All atoms other than C and H are indicated. ...
... 1) Carbon atoms are not usually shown intersections, end of each line 2) Hydrogen atoms bonded to C are not shown. They are shown in cases when they are attached to heteroatom 3) All atoms other than C and H are indicated. ...
PDF w
... I > Br > C1 N > 0 > F, which is the same as that of increasing electronegativity and of increasing hardness. For a class (a) metal ion a strong, but not complete, inversion of this order O C C U ~ S . ~The inversion can be strong enough so that for some class (a) metal ions only 0 and F complexes ca ...
... I > Br > C1 N > 0 > F, which is the same as that of increasing electronegativity and of increasing hardness. For a class (a) metal ion a strong, but not complete, inversion of this order O C C U ~ S . ~The inversion can be strong enough so that for some class (a) metal ions only 0 and F complexes ca ...
Chapter 4 Solution Chemistry
... used to determine the concentration of ions in solution. For example, Ag+ can be added to a solution of Cl- to precipitate all of the Cl- in the form of insoluble AgCl. The concentration of the Cl- is determined by weighing the AgCl and using the stoichiometry of the reaction. To determine the conce ...
... used to determine the concentration of ions in solution. For example, Ag+ can be added to a solution of Cl- to precipitate all of the Cl- in the form of insoluble AgCl. The concentration of the Cl- is determined by weighing the AgCl and using the stoichiometry of the reaction. To determine the conce ...
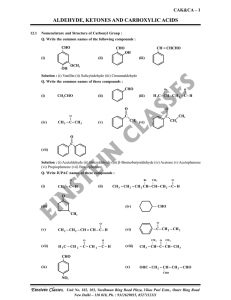
aldehyde, ketones and carboxylic acids
... Solution : Acidic hydrolysis of esters gives directly carboxylic acids while basic hydrolysis gives carboxylates, which on acidification give correspondings carboxylic acids. ...
... Solution : Acidic hydrolysis of esters gives directly carboxylic acids while basic hydrolysis gives carboxylates, which on acidification give correspondings carboxylic acids. ...
TANNIC ACID
... Consists of gallotannins obtained by solvent extraction from certain natural sources; the substance is not an acid in the chemical sense. The common name "Tannic acid" has been adopted to distinguish the commercial substance from other tannins, such as condensed tannins. These specifications relate ...
... Consists of gallotannins obtained by solvent extraction from certain natural sources; the substance is not an acid in the chemical sense. The common name "Tannic acid" has been adopted to distinguish the commercial substance from other tannins, such as condensed tannins. These specifications relate ...
chapter4-bur.2917051..
... 2 HClO4(aq) + Ca(OH)2(aq) Ca(ClO4)2(aq) + 2 H2O(l) HNO2(aq) + NaOH(aq) NaNO2(aq) + H2O(l) 2 HBr(aq) + Cu(OH)2(s) CuBr2(aq) + 2 H2O(l) The last reaction above illustrates why insoluble hydroxide compounds are considered insoluble bases. Note that ionic compounds other than hydroxides (OH-) or o ...
... 2 HClO4(aq) + Ca(OH)2(aq) Ca(ClO4)2(aq) + 2 H2O(l) HNO2(aq) + NaOH(aq) NaNO2(aq) + H2O(l) 2 HBr(aq) + Cu(OH)2(s) CuBr2(aq) + 2 H2O(l) The last reaction above illustrates why insoluble hydroxide compounds are considered insoluble bases. Note that ionic compounds other than hydroxides (OH-) or o ...
www.XtremePapers.com UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level 5070/03
... At the end of the examination, fasten all your work securely together. The number of marks is given in brackets [ ] at the end of each question or part question. ...
... At the end of the examination, fasten all your work securely together. The number of marks is given in brackets [ ] at the end of each question or part question. ...
Acid
An acid (from the Latin acidus/acēre meaning sour) is a chemical substance whose aqueous solutions are characterized by a sour taste, the ability to turn blue litmus red, and the ability to react with bases and certain metals (like calcium) to form salts. Aqueous solutions of acids have a pH of less than 7. Non-aqueous acids are usually formed when an anion (negative ion) reacts with one or more positively charged hydrogen cations. A lower pH means a higher acidity, and thus a higher concentration of positive hydrogen ions in the solution. Chemicals or substances having the property of an acid are said to be acidic.There are three common definitions for acids: the Arrhenius definition, the Brønsted-Lowry definition, and the Lewis definition. The Arrhenius definition defines acids as substances which increase the concentration of hydrogen ions (H+), or more accurately, hydronium ions (H3O+), when dissolved in water. The Brønsted-Lowry definition is an expansion: an acid is a substance which can act as a proton donor. By this definition, any compound which can easily be deprotonated can be considered an acid. Examples include alcohols and amines which contain O-H or N-H fragments. A Lewis acid is a substance that can accept a pair of electrons to form a covalent bond. Examples of Lewis acids include all metal cations, and electron-deficient molecules such as boron trifluoride and aluminium trichloride.Common examples of acids include hydrochloric acid (a solution of hydrogen chloride which is found in gastric acid in the stomach and activates digestive enzymes), acetic acid (vinegar is a dilute solution of this liquid), sulfuric acid (used in car batteries), and tartaric acid (a solid used in baking). As these examples show, acids can be solutions or pure substances, and can be derived from solids, liquids, or gases. Strong acids and some concentrated weak acids are corrosive, but there are exceptions such as carboranes and boric acid.