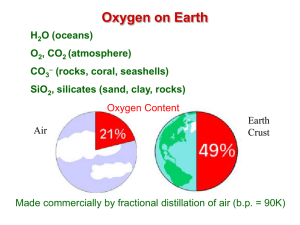
ChemChapter_7sec1_and_section2[1]FORMULA
... that give off hydrogen ions (H1+) when dissolved in water (the Arrhenius definition) Will start the formula with H. There will always be some Hydrogen next to an anion. The anion determines the name. ...
... that give off hydrogen ions (H1+) when dissolved in water (the Arrhenius definition) Will start the formula with H. There will always be some Hydrogen next to an anion. The anion determines the name. ...
Chemistry 1B General Chemistry Laboratory
... include units. Units are shown in the columns with their labels. Don’t forget your units when showing an example calculation. Since many calculations are repetitive, you can continue to work them out on a separate piece of white paper to include with your report. Observations: These are a crucial pa ...
... include units. Units are shown in the columns with their labels. Don’t forget your units when showing an example calculation. Since many calculations are repetitive, you can continue to work them out on a separate piece of white paper to include with your report. Observations: These are a crucial pa ...
Chem152
... is the empirical formula of the product? A) VO B) V2O3 C) V2O5 D) V3O2 E) V5O2 48. Fructose is a sugar found in fruit and honey. Calculate the empirical formula for fructose given its percent composition: 40.00% C, 6.72% H, and 53.29% O. A) CHO B) CH2O C) CHO2 D) C3H6O3 E) C6HO8 49. What is the mole ...
... is the empirical formula of the product? A) VO B) V2O3 C) V2O5 D) V3O2 E) V5O2 48. Fructose is a sugar found in fruit and honey. Calculate the empirical formula for fructose given its percent composition: 40.00% C, 6.72% H, and 53.29% O. A) CHO B) CH2O C) CHO2 D) C3H6O3 E) C6HO8 49. What is the mole ...
PDF document
... as HPLC, insoluble additives should be removed to prevent the columns from becoming blocked. Also, some HPLC methods for ASA determination using on-line solid-phase extraction and post-column photochemical derivatization. For the GLC methods, chemical derivatization is essential. Fluorimetric method ...
... as HPLC, insoluble additives should be removed to prevent the columns from becoming blocked. Also, some HPLC methods for ASA determination using on-line solid-phase extraction and post-column photochemical derivatization. For the GLC methods, chemical derivatization is essential. Fluorimetric method ...
Derivatization reagents
... ● Purified, dried and packaged under nitrogen in convenient 50mL Hypo-Vial Sample Storage Vials ● Supplied with elastomer septa, allowing immediate access to the sample without exposure to moisture and oxygen ● Use polar solvents (acetonitrile, dimethylformamide, dimethylsulfoxide, pyridine, tetrahy ...
... ● Purified, dried and packaged under nitrogen in convenient 50mL Hypo-Vial Sample Storage Vials ● Supplied with elastomer septa, allowing immediate access to the sample without exposure to moisture and oxygen ● Use polar solvents (acetonitrile, dimethylformamide, dimethylsulfoxide, pyridine, tetrahy ...
IB Chemistry Review. Unit I. Topics 2
... 16. 8.5 g of NH3 are dissolved in H2O to prepare a 500 cm3 solution. Which statements are correct? I. NH3 is the solute and H2O is the solution II. The concentration of the solution is 17 g dm-3 III. [NH3] = 1.0 mol dm-3 A. I and II only B. I and III only C. II and III only D. I, II and III 17. What ...
... 16. 8.5 g of NH3 are dissolved in H2O to prepare a 500 cm3 solution. Which statements are correct? I. NH3 is the solute and H2O is the solution II. The concentration of the solution is 17 g dm-3 III. [NH3] = 1.0 mol dm-3 A. I and II only B. I and III only C. II and III only D. I, II and III 17. What ...
File
... results based on data, lab safety, experimental errors Organic – basic naming, reactions, isomerism of simple organic molecules, structure, nomenclature, chemical properties Chemical Reactions - Chemical reactivity and products of chemical reaction Significant figures/Mental Math Mixtures - just sto ...
... results based on data, lab safety, experimental errors Organic – basic naming, reactions, isomerism of simple organic molecules, structure, nomenclature, chemical properties Chemical Reactions - Chemical reactivity and products of chemical reaction Significant figures/Mental Math Mixtures - just sto ...
Topic 1 Review - Capital High School
... 16. 8.5 g of NH3 are dissolved in H2O to prepare a 500 cm3 solution. Which statements are correct? I. NH3 is the solute and H2O is the solution II. The concentration of the solution is 17 g dm-3 III. [NH3] = 1.0 mol dm-3 A. I and II only B. I and III only C. II and III only D. I, II and III 17. What ...
... 16. 8.5 g of NH3 are dissolved in H2O to prepare a 500 cm3 solution. Which statements are correct? I. NH3 is the solute and H2O is the solution II. The concentration of the solution is 17 g dm-3 III. [NH3] = 1.0 mol dm-3 A. I and II only B. I and III only C. II and III only D. I, II and III 17. What ...
Formulation - Good Hope School
... [4. In the 1970s, the effects of pollutants from power stations were being seen in the lakes of Britain and Scandinavia. The problem was due to sulphur dioxide produced, form acid rain, which contains sulphuric acid. The problem became so great that in many lakes all the fish died. In North Wales, ...
... [4. In the 1970s, the effects of pollutants from power stations were being seen in the lakes of Britain and Scandinavia. The problem was due to sulphur dioxide produced, form acid rain, which contains sulphuric acid. The problem became so great that in many lakes all the fish died. In North Wales, ...
problems - chem.msu.su
... molecules. The oxidation number of this element is growing in a row С, D, E; moreover the oxidation number of this element is the same in А and С compounds. The synthesis of С from simple compound F and mercury (II) oxide is one of the few methods to obtain compound С in a pure state. The compound Y ...
... molecules. The oxidation number of this element is growing in a row С, D, E; moreover the oxidation number of this element is the same in А and С compounds. The synthesis of С from simple compound F and mercury (II) oxide is one of the few methods to obtain compound С in a pure state. The compound Y ...
Interaction Studies of Dilute Aqueous Oxalic Acid
... 0.9942 gm cm-3 at 298 K and 308 K respectively. The densities of solutions were determined from the mass of solution in the pyknometer after reaching thermal equilibrium with a thermostatically controlled water bath capable of maintaining the temperature constant ± 0.01 0C. A Citizen make electronic ...
... 0.9942 gm cm-3 at 298 K and 308 K respectively. The densities of solutions were determined from the mass of solution in the pyknometer after reaching thermal equilibrium with a thermostatically controlled water bath capable of maintaining the temperature constant ± 0.01 0C. A Citizen make electronic ...
Question paper - Unit A173/02 - Module C7 - Higher tier (PDF
... Peter is testing the food colourings in soft drinks made by different companies. He wants to find out if any of these drinks contains a banned dye chemical. Peter uses paper chromatography to separate and identify the dye chemicals in the soft drinks. Here is Peter’s chromatogram for one of the soft ...
... Peter is testing the food colourings in soft drinks made by different companies. He wants to find out if any of these drinks contains a banned dye chemical. Peter uses paper chromatography to separate and identify the dye chemicals in the soft drinks. Here is Peter’s chromatogram for one of the soft ...
International Journal of
... Benzoxazole is a heterocyclic organic compound that has benzene fused with oxazole ring containing one oxygen atom and one nitrogen atom. It is a clear to yellowish low melting solid, insoluble in water. Benzoxazole finds use in research as a starting material for the s ...
... Benzoxazole is a heterocyclic organic compound that has benzene fused with oxazole ring containing one oxygen atom and one nitrogen atom. It is a clear to yellowish low melting solid, insoluble in water. Benzoxazole finds use in research as a starting material for the s ...
Experiment 9
... QUESTIONS AND PROBLEMS A metal hydride contains 2.02 g of hydrogen and 13.88 g of metal. Calculate equivalent mass of the metal. While 53.96 g of a metal is oxidized 101.96 g of an oxide is formed. Calculate equivalent mass of the metal. 4.80 g of Ca and 7.85 g of Zn replace the same amount of hydro ...
... QUESTIONS AND PROBLEMS A metal hydride contains 2.02 g of hydrogen and 13.88 g of metal. Calculate equivalent mass of the metal. While 53.96 g of a metal is oxidized 101.96 g of an oxide is formed. Calculate equivalent mass of the metal. 4.80 g of Ca and 7.85 g of Zn replace the same amount of hydro ...
AP Chem Summer Assignment KEY
... Practice with Nomenclature, Balancing Equations, Oxidation Numbers, Solubility Rules and Problem Solving Nomenclature: Simple Inorganic Formulas and Nomenclature – Complete Exercise 1 in the Appendix of this packet. Review the naming rules and commit the naming prefixes to memory! Oxidation Numbers: ...
... Practice with Nomenclature, Balancing Equations, Oxidation Numbers, Solubility Rules and Problem Solving Nomenclature: Simple Inorganic Formulas and Nomenclature – Complete Exercise 1 in the Appendix of this packet. Review the naming rules and commit the naming prefixes to memory! Oxidation Numbers: ...
2011-2012 Paper 1
... 12. Solution A is an aqueous solution with pH 3 while solution B is an aqueous solution with pH 1. How does the [H+(aq)] in solution A compare with that in solution B? A. The [H+(aq)] in solution A is three times greater than the [H+(aq)] in solution B. B. The [H+(aq)] in solution A is one third of ...
... 12. Solution A is an aqueous solution with pH 3 while solution B is an aqueous solution with pH 1. How does the [H+(aq)] in solution A compare with that in solution B? A. The [H+(aq)] in solution A is three times greater than the [H+(aq)] in solution B. B. The [H+(aq)] in solution A is one third of ...
Part One: Ions in Aqueous Solution A. Electrolytes and Non
... Titration = process in which a solution of one reactant (the titrant) is carefully added to a solution of another reactant. Volume of titrant required for complete reaction is ...
... Titration = process in which a solution of one reactant (the titrant) is carefully added to a solution of another reactant. Volume of titrant required for complete reaction is ...
Acid
An acid (from the Latin acidus/acēre meaning sour) is a chemical substance whose aqueous solutions are characterized by a sour taste, the ability to turn blue litmus red, and the ability to react with bases and certain metals (like calcium) to form salts. Aqueous solutions of acids have a pH of less than 7. Non-aqueous acids are usually formed when an anion (negative ion) reacts with one or more positively charged hydrogen cations. A lower pH means a higher acidity, and thus a higher concentration of positive hydrogen ions in the solution. Chemicals or substances having the property of an acid are said to be acidic.There are three common definitions for acids: the Arrhenius definition, the Brønsted-Lowry definition, and the Lewis definition. The Arrhenius definition defines acids as substances which increase the concentration of hydrogen ions (H+), or more accurately, hydronium ions (H3O+), when dissolved in water. The Brønsted-Lowry definition is an expansion: an acid is a substance which can act as a proton donor. By this definition, any compound which can easily be deprotonated can be considered an acid. Examples include alcohols and amines which contain O-H or N-H fragments. A Lewis acid is a substance that can accept a pair of electrons to form a covalent bond. Examples of Lewis acids include all metal cations, and electron-deficient molecules such as boron trifluoride and aluminium trichloride.Common examples of acids include hydrochloric acid (a solution of hydrogen chloride which is found in gastric acid in the stomach and activates digestive enzymes), acetic acid (vinegar is a dilute solution of this liquid), sulfuric acid (used in car batteries), and tartaric acid (a solid used in baking). As these examples show, acids can be solutions or pure substances, and can be derived from solids, liquids, or gases. Strong acids and some concentrated weak acids are corrosive, but there are exceptions such as carboranes and boric acid.
![ChemChapter_7sec1_and_section2[1]FORMULA](http://s1.studyres.com/store/data/000546743_1-278f96ccbbfd49e292510ec017e27124-300x300.png)