Chapter 16 – Amines and Amides
... two hydrogen atoms bound to the nitrogen are called simple amides. Those with one or two aliphatic or aromatic groups are called amides of amines. The hydrogen bonding in these molecules is amongst the strongest observed. (See the table in the margin of p. 487 of your textbook.) A result is that the ...
... two hydrogen atoms bound to the nitrogen are called simple amides. Those with one or two aliphatic or aromatic groups are called amides of amines. The hydrogen bonding in these molecules is amongst the strongest observed. (See the table in the margin of p. 487 of your textbook.) A result is that the ...
Document
... Draw the hydrogen boning interaction for 1) CH3OH, 2) CH3NH2, 3) CH3CO2H, 4) HF 5) RCONHR’ Explain the boiling point of EtOH is higher than MeOMe. Which compound in the following to have the higher boiling point? 1) CH3CH2CH2CH2OH and CH3CH2OCH2CH3; 2) (CH3)3N and CH3CH2NHCH3; 3) CH3CH2CH2CH2OH and ...
... Draw the hydrogen boning interaction for 1) CH3OH, 2) CH3NH2, 3) CH3CO2H, 4) HF 5) RCONHR’ Explain the boiling point of EtOH is higher than MeOMe. Which compound in the following to have the higher boiling point? 1) CH3CH2CH2CH2OH and CH3CH2OCH2CH3; 2) (CH3)3N and CH3CH2NHCH3; 3) CH3CH2CH2CH2OH and ...
Dadkhah and Najmabadi1
... effective on Salmonella Typhimurium bacteria growth and most of the inhibition seemed to originate from other compounds, such as organic acids which is not available in strawberry extract (Puupponen-Pimia et al. 2004). Results showed that strawberry extracts clearly showed that phenolic compounds, e ...
... effective on Salmonella Typhimurium bacteria growth and most of the inhibition seemed to originate from other compounds, such as organic acids which is not available in strawberry extract (Puupponen-Pimia et al. 2004). Results showed that strawberry extracts clearly showed that phenolic compounds, e ...
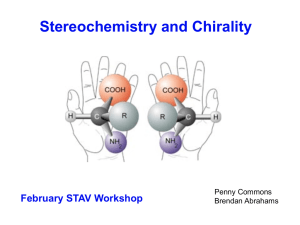
Stereochemistry of organic compounds
... • Diastereomers differ from enantiomers in that the latter are pairs of stereoisomers that differ in all stereocenters and are therefore mirror images of one another. Enantiomers of a compound with more than one stereocenter are also Diastereomers of the other stereoisomers of that compound that ar ...
... • Diastereomers differ from enantiomers in that the latter are pairs of stereoisomers that differ in all stereocenters and are therefore mirror images of one another. Enantiomers of a compound with more than one stereocenter are also Diastereomers of the other stereoisomers of that compound that ar ...
JNTUA JAWAHARLAL NEHRU TECHNOLOGICAL UNIVERSITY
... : Preparation of acetonedicarboxylic acid from citric acid 6. Condensation : Preparation of dibenzalaetone from benzaldehyde B. Identification of the following organic compounds by systematic qualitative analysis including acidic/basic/neutral character, aromatic/aliphatic, saturated/unsaturated, te ...
... : Preparation of acetonedicarboxylic acid from citric acid 6. Condensation : Preparation of dibenzalaetone from benzaldehyde B. Identification of the following organic compounds by systematic qualitative analysis including acidic/basic/neutral character, aromatic/aliphatic, saturated/unsaturated, te ...
Application of IBX Method for the Synthesis of Ketones from
... the preparation of (5) and the crude product was purified by column chromotography with ethylacetate: hexane (3:2) to give 6.24 g (30 mmol) (81%) of 1-(4-methoxyphenyl)-4-methyl-3-pentanol (5) as colourless ...
... the preparation of (5) and the crude product was purified by column chromotography with ethylacetate: hexane (3:2) to give 6.24 g (30 mmol) (81%) of 1-(4-methoxyphenyl)-4-methyl-3-pentanol (5) as colourless ...
Oxidation Reactions
... as the carboxylic acid or as the sodium benzoate salt. This compound is most effective when added to acidic foods such as fruit juices and soft drinks. The major industrial source of benzoic acid is the partial oxidation of toluene with oxygen. The process is inexpensive and environmentally benign. ...
... as the carboxylic acid or as the sodium benzoate salt. This compound is most effective when added to acidic foods such as fruit juices and soft drinks. The major industrial source of benzoic acid is the partial oxidation of toluene with oxygen. The process is inexpensive and environmentally benign. ...
Chapter 4
... Because it also has a carboxyl group, glycine is both an amine and a carboxylic acid; compounds with both groups are called amino acids. ...
... Because it also has a carboxyl group, glycine is both an amine and a carboxylic acid; compounds with both groups are called amino acids. ...
File - Pedersen Science
... Because it also has a carboxyl group, glycine is both an amine and a carboxylic acid; compounds with both groups are called amino acids. ...
... Because it also has a carboxyl group, glycine is both an amine and a carboxylic acid; compounds with both groups are called amino acids. ...
Chapter 4
... Because it also has a carboxyl group, glycine is both an amine and a carboxylic acid; compounds with both groups are called amino acids. ...
... Because it also has a carboxyl group, glycine is both an amine and a carboxylic acid; compounds with both groups are called amino acids. ...
Course No - Chemistry
... Alcohols:- Classification. Methods of formation of monohydric alcohols through reduction of aldehydes, ketones, carboxylic acids and esters using different reducing agents including mechanistic details of the reactions involved. Reactions of alcohols including Pinacole-Pinacolone rearrangement with ...
... Alcohols:- Classification. Methods of formation of monohydric alcohols through reduction of aldehydes, ketones, carboxylic acids and esters using different reducing agents including mechanistic details of the reactions involved. Reactions of alcohols including Pinacole-Pinacolone rearrangement with ...
oHi"l\-`NH / 1 \QQE
... O—O—CHz—CHz-C Hi—cm~0-o O~CH2——CH—N/l a H: H: \ H2 sure of 0.1 mm. mercury. It constitutes a color- ...
... O—O—CHz—CHz-C Hi—cm~0-o O~CH2——CH—N/l a H: H: \ H2 sure of 0.1 mm. mercury. It constitutes a color- ...
Synthesis of Several Esters
... 5. Place the labeled test tubes into the hot water bath (80-95 oC) for 10 minutes. Halfway through the heating, mix each of the solutions again by carefully removing each test tube one at a time, flicking the bottom, and replacing it. If the water in the beaker or any of the solutions in the test t ...
... 5. Place the labeled test tubes into the hot water bath (80-95 oC) for 10 minutes. Halfway through the heating, mix each of the solutions again by carefully removing each test tube one at a time, flicking the bottom, and replacing it. If the water in the beaker or any of the solutions in the test t ...
Chlorine chemistry revised 28 Jan 2017
... •Gastric juices of stomach of human beings have 0.3 to 0.4 % hydrochloric acid. White blood cells kill bacteria by producing HClO (hypochlorus acid) inside their cells. •Naturally occurring chlorine is a mixture of its two stable isotopes 35Cl and 37Cl with natural abundances of 75.8% and 24.3% resp ...
... •Gastric juices of stomach of human beings have 0.3 to 0.4 % hydrochloric acid. White blood cells kill bacteria by producing HClO (hypochlorus acid) inside their cells. •Naturally occurring chlorine is a mixture of its two stable isotopes 35Cl and 37Cl with natural abundances of 75.8% and 24.3% resp ...
Asymmetry and Stereoisomers
... • IUPAC systematic naming of organic compounds up to C8 with no more than two functional groups for a molecule, limited to non-cyclic hydrocarbons, haloalkanes, primary amines, alcohols (primary, secondary, tertiary), carboxylic acids and non-branched esters. ...
... • IUPAC systematic naming of organic compounds up to C8 with no more than two functional groups for a molecule, limited to non-cyclic hydrocarbons, haloalkanes, primary amines, alcohols (primary, secondary, tertiary), carboxylic acids and non-branched esters. ...
Chapter 19: Carboxylic Acid Derivatives
... 19.7: Physical Properties of Esters. (please read) 19.8: Reactions of Esters: A Review and a Preview. Nucleophilic acyl substitution reactions of esters (Table 19.4). Esters are less reactive toward nucleophilic acyl substitution than acid chlorides or acid anhydrides. 1. Aminolysis (Ch.19.11): Est ...
... 19.7: Physical Properties of Esters. (please read) 19.8: Reactions of Esters: A Review and a Preview. Nucleophilic acyl substitution reactions of esters (Table 19.4). Esters are less reactive toward nucleophilic acyl substitution than acid chlorides or acid anhydrides. 1. Aminolysis (Ch.19.11): Est ...
19.19 Summary
... Lithium aluminum hydride reduces esters to alcohols. Two alcohols are formed; the acyl group is reduced to the primary alcohol. ...
... Lithium aluminum hydride reduces esters to alcohols. Two alcohols are formed; the acyl group is reduced to the primary alcohol. ...
4888 Journal of the American Chemical Society 1OO:lS 1 July 19
... gave vinyl sulfone 8 which was 89 f 2% dl (8b) by 'H N M R analysis. Base-catalyzed elimination (EtOH, EtOK) yielded vinyl sulfone which was 21% d1.12The oxidative elimination, therefore, proceeds with syn stereochemistry. W e propose a n iodoso compound as an intermediate along the reaction pathway ...
... gave vinyl sulfone 8 which was 89 f 2% dl (8b) by 'H N M R analysis. Base-catalyzed elimination (EtOH, EtOK) yielded vinyl sulfone which was 21% d1.12The oxidative elimination, therefore, proceeds with syn stereochemistry. W e propose a n iodoso compound as an intermediate along the reaction pathway ...
Ch. 14 Alcohols, Ethers, & Thiols
... • They also have the ability to hydrogen bond. • These factors lead to higher B.P’s, M.P’s. etc ...
... • They also have the ability to hydrogen bond. • These factors lead to higher B.P’s, M.P’s. etc ...
Making Scents of Esters
... compounds also contain hydrogen. Those compounds that consist solely of carbon and hydrogen are called hydrocarbons. Butane, C4H10, is an example of a hydrocarbon. Other organic compounds may contain oxygen, nitrogen, sulfur, phosphorus, or one of the halogens. These groups of atoms containing eleme ...
... compounds also contain hydrogen. Those compounds that consist solely of carbon and hydrogen are called hydrocarbons. Butane, C4H10, is an example of a hydrocarbon. Other organic compounds may contain oxygen, nitrogen, sulfur, phosphorus, or one of the halogens. These groups of atoms containing eleme ...
Organic Spectroscopy UV - Ultraviolet-Visible Spectroscopy
... Ethene has lmax at 171 nm and 1,3-butadiene has lmax at 217 nm The longer the conjugated system, the smaller the energy difference between the HOMO and the LUMO A smaller energy gap results in longer !max in the ultraviolet -visible spectrum #-Carotene has 11 conjugated double bonds and an absorbanc ...
... Ethene has lmax at 171 nm and 1,3-butadiene has lmax at 217 nm The longer the conjugated system, the smaller the energy difference between the HOMO and the LUMO A smaller energy gap results in longer !max in the ultraviolet -visible spectrum #-Carotene has 11 conjugated double bonds and an absorbanc ...
Hydrocarbon Derivatives:
... Hydrocarbons • Contain only carbon & hydrogen • But carbon can also form strong covalent bonds with other elements such as: O, N, F, Cl, Br, I, S, & P ...
... Hydrocarbons • Contain only carbon & hydrogen • But carbon can also form strong covalent bonds with other elements such as: O, N, F, Cl, Br, I, S, & P ...
alcohols - profpaz.com
... · This rule is summarized as Saytzeff’s rule, which states that during intramolecular dehydration, if there is a choice of positions for the carboncarbon double bond, the preferred location is the one that generally gives the more highly substituted alkene – that is, the alkene with the most al ...
... · This rule is summarized as Saytzeff’s rule, which states that during intramolecular dehydration, if there is a choice of positions for the carboncarbon double bond, the preferred location is the one that generally gives the more highly substituted alkene – that is, the alkene with the most al ...
Phenols

In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group (—OH) bonded directly to an aromatic hydrocarbon group. The simplest of the class is phenol, which is also called carbolic acid C6H5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.Synonyms are arenols or aryl alcohols.Phenolic compounds are synthesized industrially; they also are produced by plants and microorganisms, with variation between and within species.Although similar to alcohols, phenols have unique properties and are not classified as alcohols (since the hydroxyl group is not bonded to a saturated carbon atom). They have higher acidities due to the aromatic ring's tight coupling with the oxygen and a relatively loose bond between the oxygen and hydrogen. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12).Loss of a positive hydrogen ion (H+) from the hydroxyl group of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides, although the term aryloxides is preferred according to the IUPAC Gold Book. Phenols can have two or more hydroxy groups bonded to the aromatic ring(s) in the same molecule. The simplest examples are the three benzenediols, each having two hydroxy groups on a benzene ring.Organisms that synthesize phenolic compounds do so in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research.ref name=Klepacka Some phenols are germicidal and are used in formulating disinfectants. Others possess estrogenic or endocrine disrupting activity.