(Organic Chemistry II) Pahlavan
... Organic Chemistry is the study of compounds containing carbon. There are millions of organic compounds known because carbon atom has the unique ability to bond to other carbon atoms to from large molecules. In these compounds, carbon may be bonded to other carbons via single bonds, double bonds, and ...
... Organic Chemistry is the study of compounds containing carbon. There are millions of organic compounds known because carbon atom has the unique ability to bond to other carbon atoms to from large molecules. In these compounds, carbon may be bonded to other carbons via single bonds, double bonds, and ...
Reactions of Oxacyclopropanes
... Thiols are less hydrogen-bonded and more acidic than alcohols. Compared to oxygen, sulfur has a large size, diffuse orbitals and a relatively ...
... Thiols are less hydrogen-bonded and more acidic than alcohols. Compared to oxygen, sulfur has a large size, diffuse orbitals and a relatively ...
Hydrocarbons
... oxidised to create carboxylic acids. We can use acidified dichromate or acidified permanganate as oxidising agents Elimination – As you might have guessed elimination reactions remove parts of the alcohol. In this case we remove the –OH group and one other hydrogen using concentrated sulfuric acid. ...
... oxidised to create carboxylic acids. We can use acidified dichromate or acidified permanganate as oxidising agents Elimination – As you might have guessed elimination reactions remove parts of the alcohol. In this case we remove the –OH group and one other hydrogen using concentrated sulfuric acid. ...
Carboxylic Acid Nomenclature
... 18.4: Acidity of Carboxylic Acids. The pKa of carboxylic acids typically ~ 5. They are significantly more acidic than water or alcohols. Bronsted Acidity (Ch. 1.14): Carboxylic acids transfer a proton to water to give H3O+ and carboxylate anions, RCO2 ...
... 18.4: Acidity of Carboxylic Acids. The pKa of carboxylic acids typically ~ 5. They are significantly more acidic than water or alcohols. Bronsted Acidity (Ch. 1.14): Carboxylic acids transfer a proton to water to give H3O+ and carboxylate anions, RCO2 ...
Ch.17
... Polyfunctional acids - contain other functional groups Priority for naming compounds (by functional group) 1. Carboxyl 5. Alkene 2. Carbonyl 6. Alkyne a)Aldehyde 7. Alkoxy (ether) b)Ketone 8. Alkyl 3. Alcohol 9. Halogen 4. Amine* ...
... Polyfunctional acids - contain other functional groups Priority for naming compounds (by functional group) 1. Carboxyl 5. Alkene 2. Carbonyl 6. Alkyne a)Aldehyde 7. Alkoxy (ether) b)Ketone 8. Alkyl 3. Alcohol 9. Halogen 4. Amine* ...
$doc.title
... Prepared by José Laboy, MS http: www.chem.wisc.edu/areas /clc (Resource page) Reactions of Alcohols #5: Oxidation of Primary Alcohols to Aldehydes ...
... Prepared by José Laboy, MS http: www.chem.wisc.edu/areas /clc (Resource page) Reactions of Alcohols #5: Oxidation of Primary Alcohols to Aldehydes ...
alcohols (2013)
... Recall and explain the physical properties of alcohols Recall the different structural types of alcohols Recall and explain the chemical reactions of alcohols Write balanced equations representing any reactions in the section Understand how oxidation is affected by structure Recall how conditions an ...
... Recall and explain the physical properties of alcohols Recall the different structural types of alcohols Recall and explain the chemical reactions of alcohols Write balanced equations representing any reactions in the section Understand how oxidation is affected by structure Recall how conditions an ...
Chapter 20: Carboxylic Acids and Nitriles
... Carboxylic acids with more than six carbons are only slightly soluble in water, but their conjugate base salts are water-soluble ...
... Carboxylic acids with more than six carbons are only slightly soluble in water, but their conjugate base salts are water-soluble ...
Oxidation of Alcohols
... Conversion to alkyl halides (Chapter 4) 1. Reaction with hydrogen halides 2. Reaction with thionyl chloride 3. Reaction with phosphorous trihalides Acid-catalyzed dehydration to alkenes (Chapter 5) Conversion to p-toluenesulfonate esters (Chapter 8 Conversion to ethers (Chapter 15.7) Conversion to e ...
... Conversion to alkyl halides (Chapter 4) 1. Reaction with hydrogen halides 2. Reaction with thionyl chloride 3. Reaction with phosphorous trihalides Acid-catalyzed dehydration to alkenes (Chapter 5) Conversion to p-toluenesulfonate esters (Chapter 8 Conversion to ethers (Chapter 15.7) Conversion to e ...
Chapter 1 Chemical Bonding and Chemical Structure
... – Tend to be insoluble in water – MP’s relatively high – BP’s similar to molecules of similar structure and symmetry ...
... – Tend to be insoluble in water – MP’s relatively high – BP’s similar to molecules of similar structure and symmetry ...
Topic 17 specification content - A
... I can describe common uses of esters (eg in solvents, plasticisers, perfumes and food flavourings) ...
... I can describe common uses of esters (eg in solvents, plasticisers, perfumes and food flavourings) ...
Wed March 3 lecture
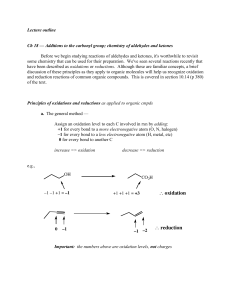
... b. The easier method for alcohols and carbonyl cmpds — again focusing only on the C(s) involved in the rxn ... oxidation => gain of bonds to O and/or loss of bonds to H reduction => loss of bonds to O and/or gain of bonds to H How does this apply to the two examples above? Does this method lead to ...
... b. The easier method for alcohols and carbonyl cmpds — again focusing only on the C(s) involved in the rxn ... oxidation => gain of bonds to O and/or loss of bonds to H reduction => loss of bonds to O and/or gain of bonds to H How does this apply to the two examples above? Does this method lead to ...
organic chem notes
... were synthesized from non-living matter. A theory known as "Vitalism" stated that a "vital force" from living organisms was necessary to make an organic compound. 1828, a German chemist Friedrich Wöhler (1800-1882) amazed the sience community by using the inorganic compound ammonium cyanate, NH4OCN ...
... were synthesized from non-living matter. A theory known as "Vitalism" stated that a "vital force" from living organisms was necessary to make an organic compound. 1828, a German chemist Friedrich Wöhler (1800-1882) amazed the sience community by using the inorganic compound ammonium cyanate, NH4OCN ...
Document
... Primary Alcohols • Primary alcohols have at least two hydrogen atoms on the carbon atom carrying the OH group. H C H ...
... Primary Alcohols • Primary alcohols have at least two hydrogen atoms on the carbon atom carrying the OH group. H C H ...
Ch 4 Carbon teacher
... thesis that life emerges from a complex combination of organic matter. The vitalistic principle goes by many names: chi or qi (China) prana (India and therapeutic touch), ki (Japan); American advocates much prefer the term energy. Many kinds of alternative therapies or energy medicines are based upo ...
... thesis that life emerges from a complex combination of organic matter. The vitalistic principle goes by many names: chi or qi (China) prana (India and therapeutic touch), ki (Japan); American advocates much prefer the term energy. Many kinds of alternative therapies or energy medicines are based upo ...
Making esters from carboxylic acids and alcohols
... to observe the smell of the esters formed. You would normally use small quantities of everything heated in a test tube stood in a hot water bath for a couple of minutes. Because the reactions are slow and reversible, you don't get a lot of ester produced in this time. The smell is often masked or di ...
... to observe the smell of the esters formed. You would normally use small quantities of everything heated in a test tube stood in a hot water bath for a couple of minutes. Because the reactions are slow and reversible, you don't get a lot of ester produced in this time. The smell is often masked or di ...
$doc.title
... Hydrolysis of Esters • Hydrolysis is cleavage of any bond by the addi4on of water molecule • Esters undergo hydrolysis reac4on in water to generate alcohol and carboxylic acid • This is the reverse ...
... Hydrolysis of Esters • Hydrolysis is cleavage of any bond by the addi4on of water molecule • Esters undergo hydrolysis reac4on in water to generate alcohol and carboxylic acid • This is the reverse ...
part 1
... Aldehydes form hydrates in water (an equilibrium) l An aldehyde hydrate is a diol and can react by the same ...
... Aldehydes form hydrates in water (an equilibrium) l An aldehyde hydrate is a diol and can react by the same ...
Chapter 15 ΠCarboxylic Acids and Esters
... or catalysts (e.g. enzymes) are present. For example, they can react with water (essentially ...
... or catalysts (e.g. enzymes) are present. For example, they can react with water (essentially ...
Back
... (b) Identify and name the functional groups in glucose which has the following structure. (b) ...
... (b) Identify and name the functional groups in glucose which has the following structure. (b) ...
CHEM 121: chapter 16
... Chapter 16:Carboxylic Acids, Esters, and Other Acid Derivatives 16.1 Structure of Carboxylic Acids and Their Derivatives 16.2 IUPAC Nomenclature for Carboxylic Acids 16.3 Common Names for Carboxylic Acids 16.4 Polyfunctional Carboxylic Acids 16.5 "Metabolic" Acids 16.6 Physical Properties of Carbox ...
... Chapter 16:Carboxylic Acids, Esters, and Other Acid Derivatives 16.1 Structure of Carboxylic Acids and Their Derivatives 16.2 IUPAC Nomenclature for Carboxylic Acids 16.3 Common Names for Carboxylic Acids 16.4 Polyfunctional Carboxylic Acids 16.5 "Metabolic" Acids 16.6 Physical Properties of Carbox ...
Silica Sulfuric Acid Promotes Aza-Michael Addition Reactions under
... organic intermediates and fine chemicals [31–34]. SSA is a strong Brønsted and Lewis acid, presumably arising from the formation of SiO2-SO3H sites on the surface. This heterogeneous catalyst can be easily separated from the reaction media, has greater selectivity, and is recyclable, easier to handl ...
... organic intermediates and fine chemicals [31–34]. SSA is a strong Brønsted and Lewis acid, presumably arising from the formation of SiO2-SO3H sites on the surface. This heterogeneous catalyst can be easily separated from the reaction media, has greater selectivity, and is recyclable, easier to handl ...
View PDF - CiteSeerX
... relates gene products that encode enzymes to the reactions these enzymes catalyze. EcoCyc also includes transport reactions and signaling pathways. 1.1 Metabolic Analysis and Nutrition-Related Studies Metabolic analysis is usually carried out through quantitative calculation of the fluxes of chemica ...
... relates gene products that encode enzymes to the reactions these enzymes catalyze. EcoCyc also includes transport reactions and signaling pathways. 1.1 Metabolic Analysis and Nutrition-Related Studies Metabolic analysis is usually carried out through quantitative calculation of the fluxes of chemica ...
Phenols

In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group (—OH) bonded directly to an aromatic hydrocarbon group. The simplest of the class is phenol, which is also called carbolic acid C6H5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.Synonyms are arenols or aryl alcohols.Phenolic compounds are synthesized industrially; they also are produced by plants and microorganisms, with variation between and within species.Although similar to alcohols, phenols have unique properties and are not classified as alcohols (since the hydroxyl group is not bonded to a saturated carbon atom). They have higher acidities due to the aromatic ring's tight coupling with the oxygen and a relatively loose bond between the oxygen and hydrogen. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12).Loss of a positive hydrogen ion (H+) from the hydroxyl group of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides, although the term aryloxides is preferred according to the IUPAC Gold Book. Phenols can have two or more hydroxy groups bonded to the aromatic ring(s) in the same molecule. The simplest examples are the three benzenediols, each having two hydroxy groups on a benzene ring.Organisms that synthesize phenolic compounds do so in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research.ref name=Klepacka Some phenols are germicidal and are used in formulating disinfectants. Others possess estrogenic or endocrine disrupting activity.