Nuggets of Knowledge for Chapter 13 – Alcohols (II)
... o As a base: the lone pair of electrons on the oxygen of an alcohol can take a hydrogen from an acid to form a protonated alcohol. This can only occur if the acid is strong enough – its pKa must be less than -2.4, the pKa of a protonated alcohol (see Ch 12 to review this concept). o As a nucleophile ...
... o As a base: the lone pair of electrons on the oxygen of an alcohol can take a hydrogen from an acid to form a protonated alcohol. This can only occur if the acid is strong enough – its pKa must be less than -2.4, the pKa of a protonated alcohol (see Ch 12 to review this concept). o As a nucleophile ...
Hydrocarbons and Fuels
... bromine reacts with the oil. Continue adding bromine water to produce a permanent yellow colour. 4. Read the burette. Subtract to find the volume of bromine water needed in the titration. 5. Repeat the experiment with: five drops of cooking oil (vegetable) and five drops of cooking oil (animal). ...
... bromine reacts with the oil. Continue adding bromine water to produce a permanent yellow colour. 4. Read the burette. Subtract to find the volume of bromine water needed in the titration. 5. Repeat the experiment with: five drops of cooking oil (vegetable) and five drops of cooking oil (animal). ...
Kekulé structure of benzene
... There are many different types of isomers, including those in which the molecules have different functional groups. Different functional groups different classes, different reactivities. E.g. C2H6O describes both an alcohol and ether ...
... There are many different types of isomers, including those in which the molecules have different functional groups. Different functional groups different classes, different reactivities. E.g. C2H6O describes both an alcohol and ether ...
Carbohydrates
... Chirality: handedness in molecules • “Handedness” is a feature that an object possesses by virtue of its symmetry. When an object has a handedness (e.g. right- or left-), its mirror image cannot be superimposed upon it (the mirror image and object are non-superimposable) • Non-superimposable means ...
... Chirality: handedness in molecules • “Handedness” is a feature that an object possesses by virtue of its symmetry. When an object has a handedness (e.g. right- or left-), its mirror image cannot be superimposed upon it (the mirror image and object are non-superimposable) • Non-superimposable means ...
Alcohols
... The body detoxifies ethanol with NAD catalyzed first by alcohol dehydrogenase (ADH) and second by aldehyde dehydrogenase (ALDH): ethanol acetic acid The reason methanol and ethylene glycol are so toxic to humans is that, when they react with NAD/ADH/ALDH, the products are more toxic than the ...
... The body detoxifies ethanol with NAD catalyzed first by alcohol dehydrogenase (ADH) and second by aldehyde dehydrogenase (ALDH): ethanol acetic acid The reason methanol and ethylene glycol are so toxic to humans is that, when they react with NAD/ADH/ALDH, the products are more toxic than the ...
EXPERIMENT 2 Properties of Alkanes, Alkenes, and Alcohols
... Reactivity with 3 M NaOH(aq). Use the general procedure for solubility tests to test only those compounds that did not dissolve in water. Use 3 M NaOH(aq) as the solvent. Watch carefully for any sign of reaction as discussed in the background. Reactivity with 3 M HCl(aq). Use the general procedure f ...
... Reactivity with 3 M NaOH(aq). Use the general procedure for solubility tests to test only those compounds that did not dissolve in water. Use 3 M NaOH(aq) as the solvent. Watch carefully for any sign of reaction as discussed in the background. Reactivity with 3 M HCl(aq). Use the general procedure f ...
A Convenient Synthesis of Amino Acid Methyl Esters
... 15]. This method has been used in the transformation of N-Boc-α-amino acids into N-unprotected αamino methyl esters [16] and some other amino acid methyl esters have been prepared using this system [17-20]. In order to demonstrate the general applicability of the method, we have examined a series of ...
... 15]. This method has been used in the transformation of N-Boc-α-amino acids into N-unprotected αamino methyl esters [16] and some other amino acid methyl esters have been prepared using this system [17-20]. In order to demonstrate the general applicability of the method, we have examined a series of ...
An algorithm to identify functional groups in organic molecules
... Methods Identification and extraction of functional groups ...
... Methods Identification and extraction of functional groups ...
Aldehydes, Ketones and Carboxylic Acids
... aldehydes the longest carbon chain is numbered starting from the carbon of the aldehyde group while in case of ketones the numbering begins from the end nearer to the carbonyl group. The substituents are prefixed in alphabetical order along with numerals indicating their positions in the carbon chai ...
... aldehydes the longest carbon chain is numbered starting from the carbon of the aldehyde group while in case of ketones the numbering begins from the end nearer to the carbonyl group. The substituents are prefixed in alphabetical order along with numerals indicating their positions in the carbon chai ...
Microsoft Word - Open Access Repository of Indian Theses
... of organic chemists since it is a valuble precursor for the synthesis of nonpeptide neurokinin NK1 receptor antagonists 43 and 44 as showed in figure III. These nonpeptidic ligands 43 and 44 are known to exhibit a variety of biological activities including neurogenic inflammation, pain transmission ...
... of organic chemists since it is a valuble precursor for the synthesis of nonpeptide neurokinin NK1 receptor antagonists 43 and 44 as showed in figure III. These nonpeptidic ligands 43 and 44 are known to exhibit a variety of biological activities including neurogenic inflammation, pain transmission ...
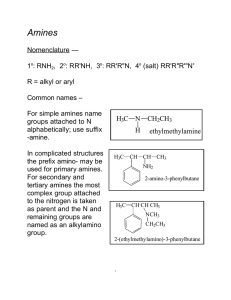
226 amines lec
... Cu + HCl free radical mechanisms. CuBr or Ar-N2 ArBr + N2 The nitrile synthesis Cu + HBr can lead to aromatic CuCN ArCN + N2 carboxylic acids via hydrolysis. ...
... Cu + HCl free radical mechanisms. CuBr or Ar-N2 ArBr + N2 The nitrile synthesis Cu + HBr can lead to aromatic CuCN ArCN + N2 carboxylic acids via hydrolysis. ...
Alcohols - Angelo State University
... hydroxyl (—OH) group is attached. The name for this chain is obtained by dropping the final -e from the name of the hydrocarbon parent name and adding the ending -ol. • Step 2. Number the longest chain to give the lowest possible number to the carbon bearing the hydroxyl ...
... hydroxyl (—OH) group is attached. The name for this chain is obtained by dropping the final -e from the name of the hydrocarbon parent name and adding the ending -ol. • Step 2. Number the longest chain to give the lowest possible number to the carbon bearing the hydroxyl ...
Aromatic Compounds
... • Quinoline, isoquinoline, indole, and purine • Quinoline, isoquinoline, and purine all contain pyridine-like nitrogens that are part of a double bond and contribute one electron to the aromatic p system • Indole and purine contain pyrrole-like nitrogens that contribute two p electrons ...
... • Quinoline, isoquinoline, indole, and purine • Quinoline, isoquinoline, and purine all contain pyridine-like nitrogens that are part of a double bond and contribute one electron to the aromatic p system • Indole and purine contain pyrrole-like nitrogens that contribute two p electrons ...
7. Organic halides
... Conformation and reactivity of cycloalkanes Experimental observations show that cyclopropane (and, to a lesser extent, cyclobutane) differ in reactivity from the larger cycloalkanes and acyclic alkanes. Cyclopropane exhibits easy ring opening (see p. 20) instead of substitution characteristic of alk ...
... Conformation and reactivity of cycloalkanes Experimental observations show that cyclopropane (and, to a lesser extent, cyclobutane) differ in reactivity from the larger cycloalkanes and acyclic alkanes. Cyclopropane exhibits easy ring opening (see p. 20) instead of substitution characteristic of alk ...
Synthesis and Structure of Alcohols
... The hydroxyl groups in alcohols can form hydrogen bonds with water, and many low molecular weight alcohols are miscible with water. Alcohols are more polar than hydrocarbons, and are better solvents for polar substances. (E.g. NaCl is partially soluble in Ethanol). ...
... The hydroxyl groups in alcohols can form hydrogen bonds with water, and many low molecular weight alcohols are miscible with water. Alcohols are more polar than hydrocarbons, and are better solvents for polar substances. (E.g. NaCl is partially soluble in Ethanol). ...
Ch 25 Hydrocarbon Compounds
... wetsuit material)), Nitrile (automotive hoses and gaskets) 1. Formed by addition polymerization 2. The resultant macromolecule is tacky and not very strong. It generally has a low melting point. 3. In order to make this stuff serviceable, it must by toughened up. This is done by a process whereby th ...
... wetsuit material)), Nitrile (automotive hoses and gaskets) 1. Formed by addition polymerization 2. The resultant macromolecule is tacky and not very strong. It generally has a low melting point. 3. In order to make this stuff serviceable, it must by toughened up. This is done by a process whereby th ...
Influence of alkyl chain length on sulfated zirconia catalysed batch
... thermal decomposition of biomass, commonly used to generate liquid bio-oil for energy applications, produces an oil of low pH due to significant levels of C1–C3 organic acids. The corrosive nature of these bio-oils is detrimental for the lifetime of deoxygenation catalysts used in their downstream r ...
... thermal decomposition of biomass, commonly used to generate liquid bio-oil for energy applications, produces an oil of low pH due to significant levels of C1–C3 organic acids. The corrosive nature of these bio-oils is detrimental for the lifetime of deoxygenation catalysts used in their downstream r ...
Lecture #13 – Introduction to Amino Acids and Proteins
... Are the building blocks put together into larger structures ? What are the physical properties of these larger structures ? How are the various types of biomolecules suited for their individual functions ? ...
... Are the building blocks put together into larger structures ? What are the physical properties of these larger structures ? How are the various types of biomolecules suited for their individual functions ? ...
1012_4th Exam_1020619 - NTOU-Chem
... 27) Define "conformation". A) Conformations are different structural representations of the same molecule. B) Conformations are different arrangements of bonds in space C) Conformations are two enantiomers. D) Conformations are all possible structural isomers for a given molecular formula E) Conform ...
... 27) Define "conformation". A) Conformations are different structural representations of the same molecule. B) Conformations are different arrangements of bonds in space C) Conformations are two enantiomers. D) Conformations are all possible structural isomers for a given molecular formula E) Conform ...
12 - Wiley
... • Carboxylic acids are polar compounds and form very strong intermolecular hydrogen bonds through both their C=O and OH groups • they have significantly higher boiling points than other types of organic compounds of comparable molecular weight • they are more soluble in water than alcohols, ethers, ...
... • Carboxylic acids are polar compounds and form very strong intermolecular hydrogen bonds through both their C=O and OH groups • they have significantly higher boiling points than other types of organic compounds of comparable molecular weight • they are more soluble in water than alcohols, ethers, ...
OXAZOLINES: THEIR SYNTHESIS AND BIOLOGICAL ACTIVITY
... The phenomenal regio- and stereochemical control in synthesis, the 2-oxazoline moiety continues to play a significant role. It has attracted organic chemists from various areas who have discovered its unique properties 5 and its capacity to serve as synthetic precursor 6 or mediator in a multitude o ...
... The phenomenal regio- and stereochemical control in synthesis, the 2-oxazoline moiety continues to play a significant role. It has attracted organic chemists from various areas who have discovered its unique properties 5 and its capacity to serve as synthetic precursor 6 or mediator in a multitude o ...
22 Acyl Substn
... e) Nitriles are different, as no leaving group is attached to electrophilic C. Nitriles do addition reactions that are analogous to the addition reactions of aldehydes and ketones. After protonation, an imine is obtained. This initial product is usually converted to other compounds by tautomerizatio ...
... e) Nitriles are different, as no leaving group is attached to electrophilic C. Nitriles do addition reactions that are analogous to the addition reactions of aldehydes and ketones. After protonation, an imine is obtained. This initial product is usually converted to other compounds by tautomerizatio ...
M_ScOrganic_Chemistr..
... Organic synthesis: Reterosynthsis, synthons and synthetic equivalents, functional group interconversion, Synthesis of amines, regiospecific, chemospecific and stereospecific reactions, umpolung methods. principles and applications of protective groups in protection of hydroxyl, amino, carbonyl and c ...
... Organic synthesis: Reterosynthsis, synthons and synthetic equivalents, functional group interconversion, Synthesis of amines, regiospecific, chemospecific and stereospecific reactions, umpolung methods. principles and applications of protective groups in protection of hydroxyl, amino, carbonyl and c ...
Derivatization Reagents - Sigma
... l It can confer volatility on substances such as carbohydrates or ...
... l It can confer volatility on substances such as carbohydrates or ...
Phenols

In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group (—OH) bonded directly to an aromatic hydrocarbon group. The simplest of the class is phenol, which is also called carbolic acid C6H5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.Synonyms are arenols or aryl alcohols.Phenolic compounds are synthesized industrially; they also are produced by plants and microorganisms, with variation between and within species.Although similar to alcohols, phenols have unique properties and are not classified as alcohols (since the hydroxyl group is not bonded to a saturated carbon atom). They have higher acidities due to the aromatic ring's tight coupling with the oxygen and a relatively loose bond between the oxygen and hydrogen. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12).Loss of a positive hydrogen ion (H+) from the hydroxyl group of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides, although the term aryloxides is preferred according to the IUPAC Gold Book. Phenols can have two or more hydroxy groups bonded to the aromatic ring(s) in the same molecule. The simplest examples are the three benzenediols, each having two hydroxy groups on a benzene ring.Organisms that synthesize phenolic compounds do so in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research.ref name=Klepacka Some phenols are germicidal and are used in formulating disinfectants. Others possess estrogenic or endocrine disrupting activity.