Chapter 15. α-AMINO ACIDS, PEPTIDES AND PROTEINS
... proteins. Recall that α-amino acids are carboxylic acids with an amino group attached to the α-carbon atom; they may be represented by the general formula RCH(NH2)COOH. Only α-amino acids will be the target of our consideration so the symbol α will further be omitted. Hydrolysis of most animal prote ...
... proteins. Recall that α-amino acids are carboxylic acids with an amino group attached to the α-carbon atom; they may be represented by the general formula RCH(NH2)COOH. Only α-amino acids will be the target of our consideration so the symbol α will further be omitted. Hydrolysis of most animal prote ...
Oxidation involving CO System ( O
... alcohols typically go to the aldehyde then acid; 2° alcohols are converted to ketone, which cannot be further converted to the acid. The aldehyde is converted back to an alcohol by alcohol (keto) reductases (reversible), however, it goes forward as the aldehyde is converted to carboxylic acid; 3° al ...
... alcohols typically go to the aldehyde then acid; 2° alcohols are converted to ketone, which cannot be further converted to the acid. The aldehyde is converted back to an alcohol by alcohol (keto) reductases (reversible), however, it goes forward as the aldehyde is converted to carboxylic acid; 3° al ...
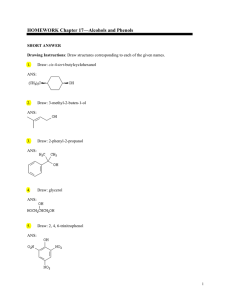
HOMEWORK Chapter 17—Alcohols and Phenols
... In E2 elimination, dehydration proceeds most readily when the two groups to be eliminated have a trans-diaxial relationship. In this compound, the only hydrogen with the proper geometric relationship to the −OH group is at C6 so the major product of this reaction is 3methylcyclohexene. ...
... In E2 elimination, dehydration proceeds most readily when the two groups to be eliminated have a trans-diaxial relationship. In this compound, the only hydrogen with the proper geometric relationship to the −OH group is at C6 so the major product of this reaction is 3methylcyclohexene. ...
Organometallic Compounds - Reagents
... Acetylide anions react with ketones and aldehydes to form a C-C bond; the product is an acetylenic (propargyl) alcohols R1 C C ...
... Acetylide anions react with ketones and aldehydes to form a C-C bond; the product is an acetylenic (propargyl) alcohols R1 C C ...
PPT File
... Naming Alcohols Common names are the name of alkyl group followed by the word “alcohol” ...
... Naming Alcohols Common names are the name of alkyl group followed by the word “alcohol” ...
chapter 4 -aromatic compounds
... • The unhybridized p orbitals must overlap to form a continuous ring of parallel orbitals. The structure must be planar (or nearly planar) for effective overlap to occur. • Delocalization of the pi electrons over the ring must lower the ...
... • The unhybridized p orbitals must overlap to form a continuous ring of parallel orbitals. The structure must be planar (or nearly planar) for effective overlap to occur. • Delocalization of the pi electrons over the ring must lower the ...
Slide 1
... • The unhybridized p orbitals must overlap to form a continuous ring of parallel orbitals. The structure must be planar (or nearly planar) for effective overlap to occur. • Delocalization of the pi electrons over the ring must lower the ...
... • The unhybridized p orbitals must overlap to form a continuous ring of parallel orbitals. The structure must be planar (or nearly planar) for effective overlap to occur. • Delocalization of the pi electrons over the ring must lower the ...
List of Objectives for Chem52
... deprotonation of alcohols/phenols to form alkoxides/phenoxides. (17.2) 4 Draw resonance structures of phenol and the phenoxide ion that involve delocalization of O lone pairs into the aromatic ring. 5 Explain why phenols are more acidic than alcohols. (The conjugate base of a phenol is resonance sta ...
... deprotonation of alcohols/phenols to form alkoxides/phenoxides. (17.2) 4 Draw resonance structures of phenol and the phenoxide ion that involve delocalization of O lone pairs into the aromatic ring. 5 Explain why phenols are more acidic than alcohols. (The conjugate base of a phenol is resonance sta ...
Grignard Reagents
... Acetylide anions react with ketones and aldehydes to form a C-C bond; the product is an acetylenic (propargyl) alcohols R1 C C ...
... Acetylide anions react with ketones and aldehydes to form a C-C bond; the product is an acetylenic (propargyl) alcohols R1 C C ...
+ :O
... esters of comparable size. Both of these types of derivatives are important, powerful donors of their acyl groups and find much use in synthesis. ...
... esters of comparable size. Both of these types of derivatives are important, powerful donors of their acyl groups and find much use in synthesis. ...
Chapter-1 ALCOHOLS
... carbonyl compounds. The choice of carbonyl type (ketone, aldehyde, ester, etc) and the type of reaction (Grignard addition or Reduction), will determine the product(s) you will get. There are primarily two types of reactions used to create alcohols from carbonyls: Grignard Addition reactions and Red ...
... carbonyl compounds. The choice of carbonyl type (ketone, aldehyde, ester, etc) and the type of reaction (Grignard addition or Reduction), will determine the product(s) you will get. There are primarily two types of reactions used to create alcohols from carbonyls: Grignard Addition reactions and Red ...
幻灯片 1 - Sun Yat-sen University
... CH3OH + HOCH3 catalyst CH3OCH3 + H2O • Nomenclature: the name for simple ethers with no or few other functional groups are a composite of the two substituents followed by ‘ether’. For example, CH3OC2H5 methyl ethyl ether, C6H5OC6H5 diphenylether. • CH3O- = methoxide ion; CH3O- = methoxyl group • Use ...
... CH3OH + HOCH3 catalyst CH3OCH3 + H2O • Nomenclature: the name for simple ethers with no or few other functional groups are a composite of the two substituents followed by ‘ether’. For example, CH3OC2H5 methyl ethyl ether, C6H5OC6H5 diphenylether. • CH3O- = methoxide ion; CH3O- = methoxyl group • Use ...
EXPERIMENT 4 (Organic Chemistry II) Pahlavan/Cherif
... Part IV. Jones Reagent ( Chromic acid test) In a small test tube, place about 1 mL acetone, 1 mL alcohol , and 2 –3 drops of the Jones reagent. Observer the color change, clear, orange ( formation of Cr +6 as CrO3 ), or blue-green (formation of Cr 3+ ). Try this test with 1-butanol, 2-butanol, 2-met ...
... Part IV. Jones Reagent ( Chromic acid test) In a small test tube, place about 1 mL acetone, 1 mL alcohol , and 2 –3 drops of the Jones reagent. Observer the color change, clear, orange ( formation of Cr +6 as CrO3 ), or blue-green (formation of Cr 3+ ). Try this test with 1-butanol, 2-butanol, 2-met ...
CARBOXYLIC ACIDS
... The carboxylate anion obtained in the ionization of aromatic carboxylic acids is best stabilized when there are electronwithdrawing substituents attached to the aromatic nucleus. It is for this reason that the nitrobenzoic acid derivatives, with the highly electron-withdrawing nitro group, are stron ...
... The carboxylate anion obtained in the ionization of aromatic carboxylic acids is best stabilized when there are electronwithdrawing substituents attached to the aromatic nucleus. It is for this reason that the nitrobenzoic acid derivatives, with the highly electron-withdrawing nitro group, are stron ...
Chapter 25 Organic and Biological Chemistry
... do not undergo addition reactions; they undergo substitution. • In substitution reactions, hydrogen is replaced by a substituent. Organic and Biological Chemistry ...
... do not undergo addition reactions; they undergo substitution. • In substitution reactions, hydrogen is replaced by a substituent. Organic and Biological Chemistry ...
Ultimate Analysis - Cheresources.com
... The alkanes or saturated hydrocarbons are more chemically inert than the other hydrocarbons, but they do form derivatives in which the hydrogen may be replaced by other atoms or groups. If only one hydrogen is replaced, the resulting radical is named by changing the “–ane” of the hydrocarbon to the ...
... The alkanes or saturated hydrocarbons are more chemically inert than the other hydrocarbons, but they do form derivatives in which the hydrogen may be replaced by other atoms or groups. If only one hydrogen is replaced, the resulting radical is named by changing the “–ane” of the hydrocarbon to the ...
BIOO211 SN05 Lecture OrganicChem
... • Have different bonding arrangements (Ethyl alcohol and dimethyl ether, it is a constitutional or functional group isomerism) o Stereoisomers • Contain the same functional groups and differ only in the arrangement of atoms in space (cis trans isomerism). • Cis and trans fatty acid (unsaturated with ...
... • Have different bonding arrangements (Ethyl alcohol and dimethyl ether, it is a constitutional or functional group isomerism) o Stereoisomers • Contain the same functional groups and differ only in the arrangement of atoms in space (cis trans isomerism). • Cis and trans fatty acid (unsaturated with ...
Acidity of Alcohols
... Making ethanol by fermentation This method only applies to ethanol. You can't make any other alcohol this way. ...
... Making ethanol by fermentation This method only applies to ethanol. You can't make any other alcohol this way. ...
Chemistry of alcohols (powerpoint)
... • Recall the definition of a covalent bond • Recall the difference types of physical bonding • Be able to balance simple equations • Be able to write out structures for simple organic molecules • Understand the IUPAC nomenclature rules for simple organic compounds • Recall the chemical properties of ...
... • Recall the definition of a covalent bond • Recall the difference types of physical bonding • Be able to balance simple equations • Be able to write out structures for simple organic molecules • Understand the IUPAC nomenclature rules for simple organic compounds • Recall the chemical properties of ...
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS)
... environment and begin to reproduce. The microorganisms form an attachment to the surface of the object by secreting a slimy, glue-like substance. Biofilms can form on just about any imaginable surface: metals, plastics, natural materials (such as rocks), medical implants, kitchen counters, contact l ...
... environment and begin to reproduce. The microorganisms form an attachment to the surface of the object by secreting a slimy, glue-like substance. Biofilms can form on just about any imaginable surface: metals, plastics, natural materials (such as rocks), medical implants, kitchen counters, contact l ...
1.4 Alcohols, Ethers, and Thiols
... Naming Ethers • The IUPAC method is to add the suffix –oxy to the smaller hydrocarbon group that is bonded to the larger alkane group • A number may be required to indicate the carbon atom that the oxygen is attached to on the longer chain • A common naming system uses the names of the two hydrocar ...
... Naming Ethers • The IUPAC method is to add the suffix –oxy to the smaller hydrocarbon group that is bonded to the larger alkane group • A number may be required to indicate the carbon atom that the oxygen is attached to on the longer chain • A common naming system uses the names of the two hydrocar ...
Phenols

In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group (—OH) bonded directly to an aromatic hydrocarbon group. The simplest of the class is phenol, which is also called carbolic acid C6H5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.Synonyms are arenols or aryl alcohols.Phenolic compounds are synthesized industrially; they also are produced by plants and microorganisms, with variation between and within species.Although similar to alcohols, phenols have unique properties and are not classified as alcohols (since the hydroxyl group is not bonded to a saturated carbon atom). They have higher acidities due to the aromatic ring's tight coupling with the oxygen and a relatively loose bond between the oxygen and hydrogen. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12).Loss of a positive hydrogen ion (H+) from the hydroxyl group of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides, although the term aryloxides is preferred according to the IUPAC Gold Book. Phenols can have two or more hydroxy groups bonded to the aromatic ring(s) in the same molecule. The simplest examples are the three benzenediols, each having two hydroxy groups on a benzene ring.Organisms that synthesize phenolic compounds do so in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research.ref name=Klepacka Some phenols are germicidal and are used in formulating disinfectants. Others possess estrogenic or endocrine disrupting activity.