Oxidation catalytic system and oxidation process using the same
... N-hydroxyphthalimide), and a co-catalyst (except phospho vanadomolybdic acid) containing an element selected from the group consisting of Group 2A elements of the Periodic Table of Elements, transition metals (Group 3A to 7A elements, Group 8 elements, Group 1B elements and Group 2B elements of the ...
... N-hydroxyphthalimide), and a co-catalyst (except phospho vanadomolybdic acid) containing an element selected from the group consisting of Group 2A elements of the Periodic Table of Elements, transition metals (Group 3A to 7A elements, Group 8 elements, Group 1B elements and Group 2B elements of the ...
INTERPRETATION OF INFRARED SPECTRA Hydrocarbons
... It should be pointed out that the C=O absorption ranges for the various carbonylcontaining functional groups overlap significantly, so it is difficult to make a definitive identification of the functional group based solely on the position of the carbonyl peak. However, most of these functional grou ...
... It should be pointed out that the C=O absorption ranges for the various carbonylcontaining functional groups overlap significantly, so it is difficult to make a definitive identification of the functional group based solely on the position of the carbonyl peak. However, most of these functional grou ...
Chapter 24. Amines
... Most amines that have 3 different substituents on N are not resolved because the molecules interconvert by pyramidal inversion ...
... Most amines that have 3 different substituents on N are not resolved because the molecules interconvert by pyramidal inversion ...
The 9-Phenyl-9-fluorenyl Group for Nitrogen Protection in
... The use of Davis’ reagent as the hydroxylating agent gives a 1:1 mixture of diastereomers under all conditions examined. N-Pf-Aspartate tert-butyl ester and free acid react with poor selectivity. Enolates generated with LiHMDS do not react at all with MoOPH [25]. The selectivity has been explained b ...
... The use of Davis’ reagent as the hydroxylating agent gives a 1:1 mixture of diastereomers under all conditions examined. N-Pf-Aspartate tert-butyl ester and free acid react with poor selectivity. Enolates generated with LiHMDS do not react at all with MoOPH [25]. The selectivity has been explained b ...
Chapter 24. Amines - Houston Community College System
... Most amines that have 3 different substituents on N are not resolved because the molecules interconvert by pyramidal inversion ...
... Most amines that have 3 different substituents on N are not resolved because the molecules interconvert by pyramidal inversion ...
Aromatic Compounds
... such as iron or tin metal or to yield an arylamine, ArNH2 • Attachment of an amino group to an aromatic ring by the two-step nitration-reduction sequence is a key part of the industrial synthesis of many dyes and pharmaceutical ...
... such as iron or tin metal or to yield an arylamine, ArNH2 • Attachment of an amino group to an aromatic ring by the two-step nitration-reduction sequence is a key part of the industrial synthesis of many dyes and pharmaceutical ...
Chapter 21: Carboxylic Acids and Their Derivatives
... Amines are more basic than alcohols, and they react with the carboxylic acid by acidbase reaction. The resulting ammonium ions do not have lone pairs, and therefore do not act as nucleophiles. This prevents the amine from reacting with the carbonyl (C=O) carbon and forming the amide. ...
... Amines are more basic than alcohols, and they react with the carboxylic acid by acidbase reaction. The resulting ammonium ions do not have lone pairs, and therefore do not act as nucleophiles. This prevents the amine from reacting with the carbonyl (C=O) carbon and forming the amide. ...
CHM 103 Lecture 22 S07
... HDPE High-density polyethylene PV Polyvinyl chloride LDPE Low-density polyethylene PP Polypropylene PS Polystyrene ...
... HDPE High-density polyethylene PV Polyvinyl chloride LDPE Low-density polyethylene PP Polypropylene PS Polystyrene ...
A Convenient Preparation of Volatile Acid Chlorides
... has been neglected for the most part. Adams and reaction mixture. The reaction is general, limUlichl have reported that they could obtain al- ited only by the volatility of the acid chloridemost quantitative yields of acid chlorides by the it has given excellent results with all the lower action of ...
... has been neglected for the most part. Adams and reaction mixture. The reaction is general, limUlichl have reported that they could obtain al- ited only by the volatility of the acid chloridemost quantitative yields of acid chlorides by the it has given excellent results with all the lower action of ...
Chapter 12: Alkanes
... able to describe the physical properties of alkanes and the products formed in the combustion and halogenation reactions of alkanes. ...
... able to describe the physical properties of alkanes and the products formed in the combustion and halogenation reactions of alkanes. ...
Chapter 12 Carboxylic Acids
... Carboxylic acids form hydrogen bonds with water, and the lower molecular-weight carboxylic acids (up through four carbon atoms) are miscible with water. As the length of the hydrocarbon chain increases, water solubility decreases until acids with more than 10 carbon atoms are essentially insoluble i ...
... Carboxylic acids form hydrogen bonds with water, and the lower molecular-weight carboxylic acids (up through four carbon atoms) are miscible with water. As the length of the hydrocarbon chain increases, water solubility decreases until acids with more than 10 carbon atoms are essentially insoluble i ...
t.h.e_2 - Homework Market
... The following sets of question pertain to intermolecular forces: (a) Provide a valid structure for each of the following molecules and then, arrange the compounds in order of increasing boiling point (you may want to consult the Aldrich catalog, an MSDS, or go to chemfinder.com to find the bps). Bri ...
... The following sets of question pertain to intermolecular forces: (a) Provide a valid structure for each of the following molecules and then, arrange the compounds in order of increasing boiling point (you may want to consult the Aldrich catalog, an MSDS, or go to chemfinder.com to find the bps). Bri ...
Organic Compounds - 2012 Book Archive
... completely in water to produce a basic solution containing Na + and OH− ions, whereas alcohols are covalent compounds that do not dissociate in water and instead form neutral aqueous solutions. You also learned that an amine (RNH 2), with its lone pairs of electrons, is a base, whereas a carboxylic ...
... completely in water to produce a basic solution containing Na + and OH− ions, whereas alcohols are covalent compounds that do not dissociate in water and instead form neutral aqueous solutions. You also learned that an amine (RNH 2), with its lone pairs of electrons, is a base, whereas a carboxylic ...
PROFESSOR SIR DEREK H. R. BARTON AND HETEROCYCLES
... In a conceptually similar approach, he developed an efficient radical deamination method based on the reaction of isocyanides with tributylstannane. The corresponding reduction of lsothiocyanldes and of isoselenocyanates also affords the deaminated products. Since the order of reactivity is tertiary ...
... In a conceptually similar approach, he developed an efficient radical deamination method based on the reaction of isocyanides with tributylstannane. The corresponding reduction of lsothiocyanldes and of isoselenocyanates also affords the deaminated products. Since the order of reactivity is tertiary ...
Chemistry 217 Problem Set 3 Recommended Problems from the Book
... 5. IR spectroscopy is commonly used by crime labs to determine the type of fiber found at a crime scene. Explain how you could use IR to distinguish between the following synthetic fibers. Note that these fibers are all polymers made up of repeating units between the brackets. Nylon contains an amid ...
... 5. IR spectroscopy is commonly used by crime labs to determine the type of fiber found at a crime scene. Explain how you could use IR to distinguish between the following synthetic fibers. Note that these fibers are all polymers made up of repeating units between the brackets. Nylon contains an amid ...
Production of NMOCs and Trace Organics During the
... among MSW components Lab-scale NMOC and HAP yields are considerably lower than regulatory estimates NMOC production is characterized by an initial “burst”, followed by much more gradual release ...
... among MSW components Lab-scale NMOC and HAP yields are considerably lower than regulatory estimates NMOC production is characterized by an initial “burst”, followed by much more gradual release ...
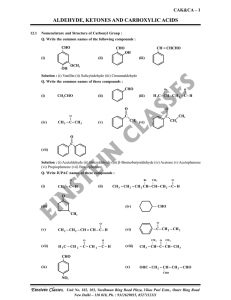
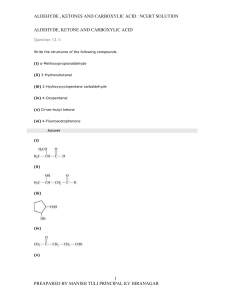
aldehyde, ketones and carboxylic acids
... Solution : A nucleophile attacks the electrophilic carbon atom of the polar carbonyl group from a direction approximately perpendicular to the plane of sp2 hybridised orbitals of carbonyl carbon. The hybridisation of carbon changes from sp2 to sp3 in this process, and a tetrahedral alkoxide intermed ...
... Solution : A nucleophile attacks the electrophilic carbon atom of the polar carbonyl group from a direction approximately perpendicular to the plane of sp2 hybridised orbitals of carbonyl carbon. The hybridisation of carbon changes from sp2 to sp3 in this process, and a tetrahedral alkoxide intermed ...
fundamentals of structure and reactivity of organic compounds
... Solution. Organic chemistry is a chemistry of carbon compounds. The properties of organic compounds are determined for the most extent by the electronic structure of the carbon atom and nature of its chemical bonds. In the excited state (1s22s12p3) carbon atom has four unpaired electrons and can the ...
... Solution. Organic chemistry is a chemistry of carbon compounds. The properties of organic compounds are determined for the most extent by the electronic structure of the carbon atom and nature of its chemical bonds. In the excited state (1s22s12p3) carbon atom has four unpaired electrons and can the ...
INORGANIC CHEMISTRY
... reported in 1859 by Hallwachs and Schafarik.Various other organometallic compounds discovered are as follows: metal carbonyls {M(CO)n} by Schützenberger in 1868; organomagnesium halides (Grignard reagents) in 1900; trimethylplatinum(IV) chloride, (CH3)3PtCl by Pope et al in 1907; bis(cyclopentadiney ...
... reported in 1859 by Hallwachs and Schafarik.Various other organometallic compounds discovered are as follows: metal carbonyls {M(CO)n} by Schützenberger in 1868; organomagnesium halides (Grignard reagents) in 1900; trimethylplatinum(IV) chloride, (CH3)3PtCl by Pope et al in 1907; bis(cyclopentadiney ...
alcohols
... Alcohols are classified into three categories. If the hydroxyl group is bonded to a terminal carbon (the chain ending carbon), that carbon will be bonded to one other carbon eg. -CH2-OH. This is a primary alcohol. If the hydroxyl is bonded to a ...
... Alcohols are classified into three categories. If the hydroxyl group is bonded to a terminal carbon (the chain ending carbon), that carbon will be bonded to one other carbon eg. -CH2-OH. This is a primary alcohol. If the hydroxyl is bonded to a ...
carbonyl compound group
... (vi) Oxime: Oximes are a class of organic compounds having the general formula RR′CNOH, where R is an organic side chain and R′ is either hydrogen or an organic side chain. If R′ is H, then it is known as aldoxime and if R′ is an organic side chain, it is known as ketoxime. ...
... (vi) Oxime: Oximes are a class of organic compounds having the general formula RR′CNOH, where R is an organic side chain and R′ is either hydrogen or an organic side chain. If R′ is H, then it is known as aldoxime and if R′ is an organic side chain, it is known as ketoxime. ...
Chapter 2- Acids and Bases
... Right Left It cannot be determined. The forward and reverse reactions are equally favored. A ...
... Right Left It cannot be determined. The forward and reverse reactions are equally favored. A ...
Aromatic electrophilic substitution
... •The affect of resonance stabilization by substituents with non-bonding electrons on reaction rates can be very large. In the case of anisole the rate of nitration is ~10,000 time faster than benzene and ~ 400 times faster then toluene. This type of ...
... •The affect of resonance stabilization by substituents with non-bonding electrons on reaction rates can be very large. In the case of anisole the rate of nitration is ~10,000 time faster than benzene and ~ 400 times faster then toluene. This type of ...
MOLECULAR REPRESENTATIONS AND INFRARED
... C=O: 1630-1780 cm-1, strong absorption If you need to use other frequencies to identify other functional groups (and sometimes you will), a table of IR frequencies will be provided. ...
... C=O: 1630-1780 cm-1, strong absorption If you need to use other frequencies to identify other functional groups (and sometimes you will), a table of IR frequencies will be provided. ...
Phenols

In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group (—OH) bonded directly to an aromatic hydrocarbon group. The simplest of the class is phenol, which is also called carbolic acid C6H5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.Synonyms are arenols or aryl alcohols.Phenolic compounds are synthesized industrially; they also are produced by plants and microorganisms, with variation between and within species.Although similar to alcohols, phenols have unique properties and are not classified as alcohols (since the hydroxyl group is not bonded to a saturated carbon atom). They have higher acidities due to the aromatic ring's tight coupling with the oxygen and a relatively loose bond between the oxygen and hydrogen. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12).Loss of a positive hydrogen ion (H+) from the hydroxyl group of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides, although the term aryloxides is preferred according to the IUPAC Gold Book. Phenols can have two or more hydroxy groups bonded to the aromatic ring(s) in the same molecule. The simplest examples are the three benzenediols, each having two hydroxy groups on a benzene ring.Organisms that synthesize phenolic compounds do so in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research.ref name=Klepacka Some phenols are germicidal and are used in formulating disinfectants. Others possess estrogenic or endocrine disrupting activity.