CHEMISTRY MOCK TEST – 1 (MEDICAL) 1 The number of gram
... Which of the following statement is not correct (a) Physical adsorption is due to Vander Waal's forces (b) Chemical adsorption decreases at high temperature and low pressure (c) Physical adsorption is reversible (d) Adsorption energy for a chemical adsorption is generally greater than that of physic ...
... Which of the following statement is not correct (a) Physical adsorption is due to Vander Waal's forces (b) Chemical adsorption decreases at high temperature and low pressure (c) Physical adsorption is reversible (d) Adsorption energy for a chemical adsorption is generally greater than that of physic ...
CHEM/ENCH 312 Transition Metal Chemistry Fall 2015
... old exams and solutions, as well as links to useful supplementary readings, etc. The Moodle site (log in with your NetID and password) can be found at: http://moodle.queensu.ca Copyright of Course Materials: The material on the course MOODLE website is copyrighted and is for the sole use of students ...
... old exams and solutions, as well as links to useful supplementary readings, etc. The Moodle site (log in with your NetID and password) can be found at: http://moodle.queensu.ca Copyright of Course Materials: The material on the course MOODLE website is copyrighted and is for the sole use of students ...
CHEM/ENCH 312 Transition Metal Chemistry Fall 2014
... old exams and solutions, as well as links to useful supplementary readings, etc. The Moodle site (log in with your NetID and password) can be found at: http://moodle.queensu.ca Copyright of Course Materials: The material on the course MOODLE website is copyrighted and is for the sole use of students ...
... old exams and solutions, as well as links to useful supplementary readings, etc. The Moodle site (log in with your NetID and password) can be found at: http://moodle.queensu.ca Copyright of Course Materials: The material on the course MOODLE website is copyrighted and is for the sole use of students ...
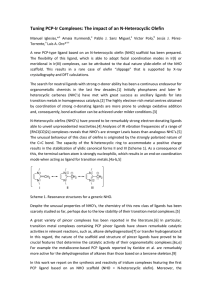
Tuning PCP-Ir Complexes: The impact of an N
... coordination mode of the ligand core (NHO) in the Ir(I) and Ir(III) complexes described here represents a rare case of olefin “slippage” that has been studied by X-ray crystallography and DFT calculations. The in situ deprotonation of 1,3-bis(2-(diphenylphosphino)ethyl)-2-methylimidazolium chloride ...
... coordination mode of the ligand core (NHO) in the Ir(I) and Ir(III) complexes described here represents a rare case of olefin “slippage” that has been studied by X-ray crystallography and DFT calculations. The in situ deprotonation of 1,3-bis(2-(diphenylphosphino)ethyl)-2-methylimidazolium chloride ...
Solutions, Solubility Rules, and Molarity File
... • A nonelectrolyte may dissolve in water, but it does not dissociate into ions when it does so. – Solutions do not conduct electricity ...
... • A nonelectrolyte may dissolve in water, but it does not dissociate into ions when it does so. – Solutions do not conduct electricity ...
Chemical Formulas
... single atom Na+ or S2- usually tell by column on periodic table, some elements have more than one oxidation number or charge Binary compounds- only 2 elements in the compound Na2S Polyatomic ions - ions formed from more than one type of atom covalently bonded together OHPO43- NH4+ ...
... single atom Na+ or S2- usually tell by column on periodic table, some elements have more than one oxidation number or charge Binary compounds- only 2 elements in the compound Na2S Polyatomic ions - ions formed from more than one type of atom covalently bonded together OHPO43- NH4+ ...
H 2 SO 4
... In its compound, fluorine is always assigned an oxidation sate of -1. Oxygen is usually assigned an oxidation of -2 in its covalent compounds, such as CO, CO2, SO2. Exceptions to this rule includes peroxides (compounds containing the O22- group), where each oxygen is assigned an oxidation state of - ...
... In its compound, fluorine is always assigned an oxidation sate of -1. Oxygen is usually assigned an oxidation of -2 in its covalent compounds, such as CO, CO2, SO2. Exceptions to this rule includes peroxides (compounds containing the O22- group), where each oxygen is assigned an oxidation state of - ...
Predicting synthesis and decomposition reactions
... (MUCH more on this later!) • A reaction in which electrons are transferred from one atom to another is called an ____________________ reaction. ...
... (MUCH more on this later!) • A reaction in which electrons are transferred from one atom to another is called an ____________________ reaction. ...
Experiment Report Form
... each MPG is coordinated to the Co(II) centers through the carboxy and the thio functions, these later having a bridging role between the two Co(II) atoms. The octahedral sphere of the metallic cation is fulfilled by 3 water molecules. The first coordination sphere of Co(II) compares surprisingly wel ...
... each MPG is coordinated to the Co(II) centers through the carboxy and the thio functions, these later having a bridging role between the two Co(II) atoms. The octahedral sphere of the metallic cation is fulfilled by 3 water molecules. The first coordination sphere of Co(II) compares surprisingly wel ...
Reactions In Aqueous Solution
... When two different aqueous solutions of ionic compounds are mixed, an insoluble solid (precipitate) can separate out of solution. To predict the products of a precipitate reaction you must know which ionic substances are insoluble in water. ...
... When two different aqueous solutions of ionic compounds are mixed, an insoluble solid (precipitate) can separate out of solution. To predict the products of a precipitate reaction you must know which ionic substances are insoluble in water. ...
Project Advance Chemistry 106 Sample Questions
... respectively. Determine the value of the Eocell for a voltaic cell in which the overall reaction is ...
... respectively. Determine the value of the Eocell for a voltaic cell in which the overall reaction is ...
Document
... ___________________________________________________________________________________ [Ti(H2O)6]3+ ...
... ___________________________________________________________________________________ [Ti(H2O)6]3+ ...
BRONSTED-LOWRY THEORY IN WATER... acid conjugate base of
... - Lewis theory treats acid-base chemistry as ELECTRON-TRANSFER chemistry involving pairs of electrons - Lewis acid-base reactions form new covalent bonds (of interest to organic chemists!) ACIDS are ACCEPTORS of electron pairs ... this is why some METAL IONS, even though they contain no hydorgen ion ...
... - Lewis theory treats acid-base chemistry as ELECTRON-TRANSFER chemistry involving pairs of electrons - Lewis acid-base reactions form new covalent bonds (of interest to organic chemists!) ACIDS are ACCEPTORS of electron pairs ... this is why some METAL IONS, even though they contain no hydorgen ion ...
Complexometric Titration
... Molecule or ion with at least 1 pair of unshared electron can form covalent bond with metal ion = ligands The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs Eg of ligands = ammonia, cyanide ions, halide ions, water (neutral/-ve charg ...
... Molecule or ion with at least 1 pair of unshared electron can form covalent bond with metal ion = ligands The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs Eg of ligands = ammonia, cyanide ions, halide ions, water (neutral/-ve charg ...
chapter 9: aqueous solutions
... 2. write the formula of the compound followed by an arrow 3. balance using coefficients 4. add state symbols (state of pure substance on the left, ((s) usually), (aq) for ions on the right) Example 1: Solid Sodium carbonate dissolves in water ...
... 2. write the formula of the compound followed by an arrow 3. balance using coefficients 4. add state symbols (state of pure substance on the left, ((s) usually), (aq) for ions on the right) Example 1: Solid Sodium carbonate dissolves in water ...
Linkage Isomers What`s going on?
... In the early 1960's, a series of experiments in the laboratories of Barnett Rosenberg at the Michigan State University found some peculiar results. An experiment designed to measure the effect of electrical currents on cell growth yielded Escherichia colithat were 300 times the normal length. This e ...
... In the early 1960's, a series of experiments in the laboratories of Barnett Rosenberg at the Michigan State University found some peculiar results. An experiment designed to measure the effect of electrical currents on cell growth yielded Escherichia colithat were 300 times the normal length. This e ...
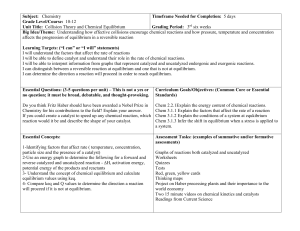
Subject:
... I can distinguish between a reversible reaction at equilibrium and one that is not at equilibrium. I can determine the direction a reaction will proceed in order to reach equilibrium. Essential Questions: (3-5 questions per unit) – This is not a yes or no question; it must be broad, debatable, and t ...
... I can distinguish between a reversible reaction at equilibrium and one that is not at equilibrium. I can determine the direction a reaction will proceed in order to reach equilibrium. Essential Questions: (3-5 questions per unit) – This is not a yes or no question; it must be broad, debatable, and t ...