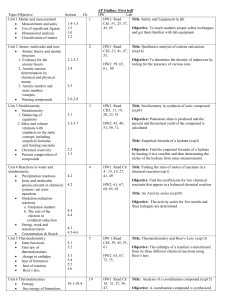
Prescribed Practicals
... § Sensors/probes eudiometer/ temperature probes/ pH § Data collection sofware probes, etc. ...
... § Sensors/probes eudiometer/ temperature probes/ pH § Data collection sofware probes, etc. ...
Conformation Switching in Gas-Phase Complexes of Histidine with
... MPW1PW91 using the Gaussian 03 package.24 The SDD basis set with a relativistic effective core potential was used for Sr and Ba atoms. For the Ca and Ba complexes, the 6-311++G(d,p) basis set was used. Corrections for zero-point vibrational energy and basis-set superposition error were included for ...
... MPW1PW91 using the Gaussian 03 package.24 The SDD basis set with a relativistic effective core potential was used for Sr and Ba atoms. For the Ca and Ba complexes, the 6-311++G(d,p) basis set was used. Corrections for zero-point vibrational energy and basis-set superposition error were included for ...
Extremely Facile Template Synthesis of Gold(III)
... [Au(L)](ClO4)3‚1/2H2O and [Au(L)Cl2]AuCl4 indicate that they both are diamagnetic. The molar magnetic susceptibility (χM) value for [Au(L)Cl2]AuCl4 is -67.11 × 10-6 emu at 292 K. It is quite unusual that the six-coordinate Au(III) complex is diamagnetic. If the complex were in a perfect octahedral g ...
... [Au(L)](ClO4)3‚1/2H2O and [Au(L)Cl2]AuCl4 indicate that they both are diamagnetic. The molar magnetic susceptibility (χM) value for [Au(L)Cl2]AuCl4 is -67.11 × 10-6 emu at 292 K. It is quite unusual that the six-coordinate Au(III) complex is diamagnetic. If the complex were in a perfect octahedral g ...
The s-Block Elements - GCG-42
... BeCl2 is essentially covalent, with comparatively low m.pt. The lower members in group II form essentially ionic chlorides, with Mg having intermediate properties. ...
... BeCl2 is essentially covalent, with comparatively low m.pt. The lower members in group II form essentially ionic chlorides, with Mg having intermediate properties. ...
Chem+174–Lecture+4c+..
... (PPh3: d= ~ -5ppm) because the phosphorus atom acts as a good s-donor and a weak s*-acceptor, which results in a net loss of electron-density on the P-atom ...
... (PPh3: d= ~ -5ppm) because the phosphorus atom acts as a good s-donor and a weak s*-acceptor, which results in a net loss of electron-density on the P-atom ...
Reversible binding of sulfur dioxide to arylplatinum (II) and nickel (II
... one of the complexes was crystallographically studied. IR and Raman Spectra. IR spectra of 2a-f (in KBr pellets or in Nujol mulls between NaCl windows) show two strong absorption bands in the region 1231-1245 and 1053-1078 cm-l, respectively, which can be assigned to the asymmetric and symmetric S-0 ...
... one of the complexes was crystallographically studied. IR and Raman Spectra. IR spectra of 2a-f (in KBr pellets or in Nujol mulls between NaCl windows) show two strong absorption bands in the region 1231-1245 and 1053-1078 cm-l, respectively, which can be assigned to the asymmetric and symmetric S-0 ...
summerpp_4
... How would you prepare 60.0 mL of 0.2 M HNO3 from a stock solution of 4.00 M HNO3? ...
... How would you prepare 60.0 mL of 0.2 M HNO3 from a stock solution of 4.00 M HNO3? ...
Analysing Acids and Bases
... The base – is a proton (H+) acceptor An acid-base reaction involves two conjugate acid-base pairs. ...
... The base – is a proton (H+) acceptor An acid-base reaction involves two conjugate acid-base pairs. ...
Abstract - Trade Science Inc
... ions formed complexes of the type [MLL-(H2O)2], where L = bromosalicylaldehyde and HL- = pentane-2,4dion, 1-phenylbutane-1,3-dione or 1,3,-diphenyl propane dione, which have been synthesized by 1:1:1 molar reactions of metal chlorides with 5-bromo salicylaldehydediketones. For continuity, we have em ...
... ions formed complexes of the type [MLL-(H2O)2], where L = bromosalicylaldehyde and HL- = pentane-2,4dion, 1-phenylbutane-1,3-dione or 1,3,-diphenyl propane dione, which have been synthesized by 1:1:1 molar reactions of metal chlorides with 5-bromo salicylaldehydediketones. For continuity, we have em ...
VanadiumNotes
... Inspection shows that, should the partial pressure of Cl2 decrease, the reaction quotient Q will become less than 1, and logQ will therefore eventually become a negative number. For example, log(0.9) = -0.046. This will cause the second term of the Nernst equation to become positive in value, and gr ...
... Inspection shows that, should the partial pressure of Cl2 decrease, the reaction quotient Q will become less than 1, and logQ will therefore eventually become a negative number. For example, log(0.9) = -0.046. This will cause the second term of the Nernst equation to become positive in value, and gr ...
Topic/Objective - cloudfront.net
... a small test tube and then heated in boiling water until all the liquid vaporizes and fills the tube as excess water escapes. After the gas is cooled, the mass, volume, and pressure is measured to determine the molecular mass of the substance using ideal gas law Title: Determination of the molar vol ...
... a small test tube and then heated in boiling water until all the liquid vaporizes and fills the tube as excess water escapes. After the gas is cooled, the mass, volume, and pressure is measured to determine the molecular mass of the substance using ideal gas law Title: Determination of the molar vol ...
Thermochemical Approaches to Neutralization Reactions between
... hydroxide ion. Assuming simply the reaction is composed of the acid dissociation of penolic compounds in aqueous solutions and the formation of water from aqueous hydrogen ion and hydroxide ion, the value of ∆rH is estimated according to the Hess’s law. The values of ∆rH evaluated from the thermoche ...
... hydroxide ion. Assuming simply the reaction is composed of the acid dissociation of penolic compounds in aqueous solutions and the formation of water from aqueous hydrogen ion and hydroxide ion, the value of ∆rH is estimated according to the Hess’s law. The values of ∆rH evaluated from the thermoche ...
Polymerization of Olefins: An Outlook After 50
... By this procedure the Ti-Et bond comes under the transinfluence of the bridged aluminum and presumably suffers weakening. This weakening is responsible for the two phenomena, polymerization and reduction. In the absence of ethylene only the reduction reaction has to be taken into account. The octah ...
... By this procedure the Ti-Et bond comes under the transinfluence of the bridged aluminum and presumably suffers weakening. This weakening is responsible for the two phenomena, polymerization and reduction. In the absence of ethylene only the reduction reaction has to be taken into account. The octah ...
Kinetics
... change, for the reaction at 25˚C. Explain your reasoning. Because the reaction’s ∆S˚ is very little and the equation to determine free energy change is ∆G˚= ∆H˚-T ∆S˚, it can be assumed that with a negative ∆H˚ and at 25˚C or 298˚K, that the reaction is spontaneous. By having a spontaneous reaction, ...
... change, for the reaction at 25˚C. Explain your reasoning. Because the reaction’s ∆S˚ is very little and the equation to determine free energy change is ∆G˚= ∆H˚-T ∆S˚, it can be assumed that with a negative ∆H˚ and at 25˚C or 298˚K, that the reaction is spontaneous. By having a spontaneous reaction, ...
ACS Practice Test 1
... They raise the boiling point of water when dissolved in it. 42. The addition of a catalyst in a chemical reaction (A) increases the concentration of products at equilibrium. (B) increases the fraction of reactant molecules with a given kinetic energy. (C) provides an alternate path with a different ...
... They raise the boiling point of water when dissolved in it. 42. The addition of a catalyst in a chemical reaction (A) increases the concentration of products at equilibrium. (B) increases the fraction of reactant molecules with a given kinetic energy. (C) provides an alternate path with a different ...