5 - Polyprotic Acids
... Subsequent dissociations will be ________________ by hydronium _____________ from the first dissociation ...
... Subsequent dissociations will be ________________ by hydronium _____________ from the first dissociation ...
fied molal concentration. The molality, or molal concentration, is the
... tend to be E°, ared, and aox. If you haven’t had physical chemistry yet, the activities ared and aox might seem like foreign concepts. Activity is just a modi- ...
... tend to be E°, ared, and aox. If you haven’t had physical chemistry yet, the activities ared and aox might seem like foreign concepts. Activity is just a modi- ...
CHEM 481. Transition Metal Complexes. Assignment 7. Answers
... elements belongs to those species with d n configurations from d 1 to d 9 in at least one common oxidation state. Thus Sc3+ and Zn2+ do not behave like typical TM, but Ti3+, Ti2+ and Ti+ , as well as Cu 2+ behave are typical. A coordination compound is a compound formed by Lewis acid/base donor acce ...
... elements belongs to those species with d n configurations from d 1 to d 9 in at least one common oxidation state. Thus Sc3+ and Zn2+ do not behave like typical TM, but Ti3+, Ti2+ and Ti+ , as well as Cu 2+ behave are typical. A coordination compound is a compound formed by Lewis acid/base donor acce ...
Advanced Placement Chemistry Test
... At equilibrium, the rate of the forward reaction is the same as the rate of the reverse reaction. At equilibrium, the number of moles of SO3 present in the reaction vessel will always be the same as the number of moles of SO2 present, regardless of the temperature. Changing the volume of the vessel ...
... At equilibrium, the rate of the forward reaction is the same as the rate of the reverse reaction. At equilibrium, the number of moles of SO3 present in the reaction vessel will always be the same as the number of moles of SO2 present, regardless of the temperature. Changing the volume of the vessel ...
Academic Year 2009/2010 Semester I KTT 212/3 Inorganic
... An overview of the background and basic aspects related to the coordination compounds or complexes which include the definition, nomenclature based on IUPAC system, coordination number, oxidation state for central metal atom, types of ligands and complexes. Establishment of different structures owin ...
... An overview of the background and basic aspects related to the coordination compounds or complexes which include the definition, nomenclature based on IUPAC system, coordination number, oxidation state for central metal atom, types of ligands and complexes. Establishment of different structures owin ...
Chapter 4
... For acid/base: moles H+ = moles OHUse an indicator to determine endpoint chemical that changes color at endpoint Record volume of second solution 3. Calculate molarity of unknown solution based on molarity and volume of 1 solution and volume recorded in titration. ...
... For acid/base: moles H+ = moles OHUse an indicator to determine endpoint chemical that changes color at endpoint Record volume of second solution 3. Calculate molarity of unknown solution based on molarity and volume of 1 solution and volume recorded in titration. ...
4.2 Chelation Therapy
... forming stable complexes through the formation of multiple chelate rings. The increase in complex stability with increasing number of chelate rings can be seen in the stability constant (b1) variation for the series of Cd21 complexes with iminodiacetic acid (10, idaH2), b1 ¼ 105.7; nitrilotriacetic ...
... forming stable complexes through the formation of multiple chelate rings. The increase in complex stability with increasing number of chelate rings can be seen in the stability constant (b1) variation for the series of Cd21 complexes with iminodiacetic acid (10, idaH2), b1 ¼ 105.7; nitrilotriacetic ...
1 | Page Chemistry Lecture #35 Chemistry Lecture #35: Names and
... Binary ionic compounds with transition metals or group 4A elements often require the use of Roman numerals since these metals have variable oxidation states. For example, CuO and Cu2O could both be called copper oxide. But the oxidation state of Cu in CuO is +2, and the oxidation state of Cu in Cu2 ...
... Binary ionic compounds with transition metals or group 4A elements often require the use of Roman numerals since these metals have variable oxidation states. For example, CuO and Cu2O could both be called copper oxide. But the oxidation state of Cu in CuO is +2, and the oxidation state of Cu in Cu2 ...
Activity sheet (RTF 136kB)
... excess forming a complex B which rapidly darkens in colour when exposed to air. The final solution contains complex C. Identify A, B and C. ...
... excess forming a complex B which rapidly darkens in colour when exposed to air. The final solution contains complex C. Identify A, B and C. ...
CATION ANALYSIS - webhosting.au.edu
... identity of an unknown sample. Given a totally “ unknown” sample, how does one go about determining what is actually present? This process is called “ qualitative analysis”. Cations are classified into five groups on the basis of their behavior against some reagents by using group reagents; we can d ...
... identity of an unknown sample. Given a totally “ unknown” sample, how does one go about determining what is actually present? This process is called “ qualitative analysis”. Cations are classified into five groups on the basis of their behavior against some reagents by using group reagents; we can d ...
Document
... Two main selection rules (a) orbital selection or Laporte selection rule (b) spin selection rule ...
... Two main selection rules (a) orbital selection or Laporte selection rule (b) spin selection rule ...
Chapter 19 d-block metal chemistry: general considerations
... number of molecules or ions called ligands surround a central metal atom or ion. Ligand – atom or ion that bonds to the central atom. Each ligand shares a pair of its electrons with the metal. Coordinate-covalent bond L: M (both electrons are from the ligand. Coordination number – The number of at ...
... number of molecules or ions called ligands surround a central metal atom or ion. Ligand – atom or ion that bonds to the central atom. Each ligand shares a pair of its electrons with the metal. Coordinate-covalent bond L: M (both electrons are from the ligand. Coordination number – The number of at ...
Lecture 7b - University of California, Los Angeles
... Metal Nitrosyl Complexes II • The second compound, an iron cyano complex ([Fe(CN)5(NO)]2-), was described about 60 years later by K. L. Playfair. • The dark-red sodium nitroprusside Na2[Fe(CN)5(NO)]*2 H2O) is obtained from potassium ferrocyanide and concentrated nitric acid • It is a potent vasodil ...
... Metal Nitrosyl Complexes II • The second compound, an iron cyano complex ([Fe(CN)5(NO)]2-), was described about 60 years later by K. L. Playfair. • The dark-red sodium nitroprusside Na2[Fe(CN)5(NO)]*2 H2O) is obtained from potassium ferrocyanide and concentrated nitric acid • It is a potent vasodil ...
... All the compounds and solvents used were purchased from Aldrich and Sigma and used as received without further purification. Elemental microanalyses of the separated ligands for C, H, and N were determined on Automatic Analyzer CHNS Vario ELIII, Germany. The 1H-NMR spectrum was obtained with a JEOL ...
2011 Lecture 22: Transport in Bulk Electrolytes
... voltage. In the NP equation, the flux is decomposed into to contributions, chemical diffusion due to concentrated gradients, and electromigration, or drift, in the electric field. Conservation of mass implies, ∂ci + ∇ · (~uci + F~i ) = Ri ∂t ...
... voltage. In the NP equation, the flux is decomposed into to contributions, chemical diffusion due to concentrated gradients, and electromigration, or drift, in the electric field. Conservation of mass implies, ∂ci + ∇ · (~uci + F~i ) = Ri ∂t ...
Document
... Thus, it takes less energy to pair up in the “t2g“ set than would be required to move up to the “eg” set The number of unpaired electrons in a Strong-Field Ligand complex is less than in the free ion Strong-Field ligands create low-spin complexes, i.e., those with fewer unpaired electrons Ge ...
... Thus, it takes less energy to pair up in the “t2g“ set than would be required to move up to the “eg” set The number of unpaired electrons in a Strong-Field Ligand complex is less than in the free ion Strong-Field ligands create low-spin complexes, i.e., those with fewer unpaired electrons Ge ...
Phosphine Complexes of the Platinum Group Metals
... It seems likely, on the basis of our present knowledge of homogeneous catalytic mechanisms, that many applicationsof hydride complexes of the platinum metals will soon emerge. For these reasons the preparation of a number of these complexes has been surveyed in the Johnson Matthey Research Laborator ...
... It seems likely, on the basis of our present knowledge of homogeneous catalytic mechanisms, that many applicationsof hydride complexes of the platinum metals will soon emerge. For these reasons the preparation of a number of these complexes has been surveyed in the Johnson Matthey Research Laborator ...
makeup6
... If the concentration of ammonia gas is tripled, the value of the equilibrium constant will (A) triple (B) increase, buy by more than a factor of three (C) decrease to one-third its value (D) remain the same 24. The weak base ionization constant (Kb) for hydroxylamine, HONH2, is 1.1 x 10¯8. Which of ...
... If the concentration of ammonia gas is tripled, the value of the equilibrium constant will (A) triple (B) increase, buy by more than a factor of three (C) decrease to one-third its value (D) remain the same 24. The weak base ionization constant (Kb) for hydroxylamine, HONH2, is 1.1 x 10¯8. Which of ...
r-Benzoin Oxime in Higher Oxidation State 3d Metal Cluster
... previously observed in other 3d metal/oximato clusters,13 a static disorder of an Hþ ion over two or more sites would average out its influence on the structural parameters and thus make it very difficult to detect. Complex 1 is the first structurally characterized 3d metal complex in the oxidation ...
... previously observed in other 3d metal/oximato clusters,13 a static disorder of an Hþ ion over two or more sites would average out its influence on the structural parameters and thus make it very difficult to detect. Complex 1 is the first structurally characterized 3d metal complex in the oxidation ...
Metal–organic complexation in the marine environment | SpringerLink
... approach for model ligands bound to Fe(III) in UV seawater. The agreement is excellent indicating that both methods give comparable results. To date the window for Kcond Fe(III)L using this method is log K 18–23. In addition to the stability constant data, the kinetic data for Fe’L (Table 1) reflect ...
... approach for model ligands bound to Fe(III) in UV seawater. The agreement is excellent indicating that both methods give comparable results. To date the window for Kcond Fe(III)L using this method is log K 18–23. In addition to the stability constant data, the kinetic data for Fe’L (Table 1) reflect ...
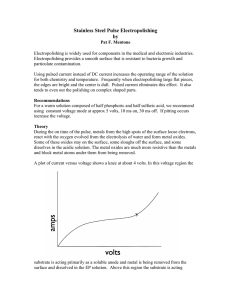
This is an overview of what can happen during the
... primarily as an insoluble anode and the water is being electrolyzed to oxygen gas and hydrogen ions. In order for polishing to occur, the current and voltage must be above the knee of the curve, this location is marked with an X in the above graph If the substrate functioned as a 100% soluble anode ...
... primarily as an insoluble anode and the water is being electrolyzed to oxygen gas and hydrogen ions. In order for polishing to occur, the current and voltage must be above the knee of the curve, this location is marked with an X in the above graph If the substrate functioned as a 100% soluble anode ...