Single crystal structure determination using synchrotron X
... TFE is known to be a weak acid and to donate a hydrogen bond, comparable to that of phenol, and a 1:1 complex of TFE and pyridine as an acid-base pair is reported. Therefore, we expected that the dissociated pyridyl moiety from the metal ion center would be stabilized by the strong hydrogen bond of ...
... TFE is known to be a weak acid and to donate a hydrogen bond, comparable to that of phenol, and a 1:1 complex of TFE and pyridine as an acid-base pair is reported. Therefore, we expected that the dissociated pyridyl moiety from the metal ion center would be stabilized by the strong hydrogen bond of ...
chemical kinetics
... understanding. For example, which parameters determine as to how rapidly food gets spoiled? How to design a rapidly setting material for dental filling? Or what controls the rate at which fuel burns in an auto engine? All these questions can be answered by the branch of chemistry, which deals with t ...
... understanding. For example, which parameters determine as to how rapidly food gets spoiled? How to design a rapidly setting material for dental filling? Or what controls the rate at which fuel burns in an auto engine? All these questions can be answered by the branch of chemistry, which deals with t ...
High-pressure experiments and modeling of methane/air catalytic
... are largely influenced by kinetics. The effectiveness of the modeling tools thus depends strongly on the availability of reliable, heterogeneous, kinetic data, which are necessary for the correct description of the catalytic processes. In gas turbine applications, the catalysts of choice are palladi ...
... are largely influenced by kinetics. The effectiveness of the modeling tools thus depends strongly on the availability of reliable, heterogeneous, kinetic data, which are necessary for the correct description of the catalytic processes. In gas turbine applications, the catalysts of choice are palladi ...
Entering and leaving group effects in Oh ligand substitutions
... e.g. rates for the following reaction decrease as L = PPh3 > PPh2Me > PPhMe2. ...
... e.g. rates for the following reaction decrease as L = PPh3 > PPh2Me > PPhMe2. ...
In Situ Soft X‑ray Absorption Spectroscopy Applied to Solid
... since the absorption peak of EtOH is observed in the energy region higher than 286.4 eV (Figure 1a), the fitting analyses have been carried out in the energy region from 284 to 286.4 eV. As a result, all of the in situ spectra, except for the energy scans of the in situ N K-edge XAS from 84 to 120 mi ...
... since the absorption peak of EtOH is observed in the energy region higher than 286.4 eV (Figure 1a), the fitting analyses have been carried out in the energy region from 284 to 286.4 eV. As a result, all of the in situ spectra, except for the energy scans of the in situ N K-edge XAS from 84 to 120 mi ...
2004 AP Chemistry Free-Response Questions Form B
... 8. The gas-phase conversion reaction between the geometric isomers cis-2-butene and trans-2-butene is represented by the equation above. The value of the equilibrium constant, Keq , for the reaction is 3.2 at 298 K and 1.0 atm. (a) In a mixture of the isomers at equilibrium at 298 K and 1.0 atm, whi ...
... 8. The gas-phase conversion reaction between the geometric isomers cis-2-butene and trans-2-butene is represented by the equation above. The value of the equilibrium constant, Keq , for the reaction is 3.2 at 298 K and 1.0 atm. (a) In a mixture of the isomers at equilibrium at 298 K and 1.0 atm, whi ...
Kinetics of the Gas-Phase Reactions of C1
... energy are found to decrease the rate constant as approximately To.8or (KE,,)4,8. The temperature dependence of reaction 1 has also been examined in a high-pressure mass spectrometry experiment (HPMS).39 Our data are compared with the HPMS temperature dependence in Figure 2. Temperature has been con ...
... energy are found to decrease the rate constant as approximately To.8or (KE,,)4,8. The temperature dependence of reaction 1 has also been examined in a high-pressure mass spectrometry experiment (HPMS).39 Our data are compared with the HPMS temperature dependence in Figure 2. Temperature has been con ...
Condensed Matter 2
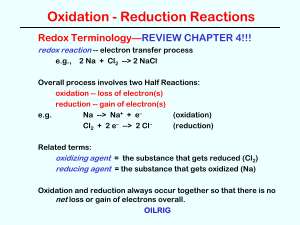
... all reactants, the reaction is not complete, and the reaction is reversible. A + B = C + D In principle, all chemical reactions are reversible, but this reversibility may not be observable if the fraction of products in the equilibrium mixture is very small, or if the reverse reaction is kinetic ...
... all reactants, the reaction is not complete, and the reaction is reversible. A + B = C + D In principle, all chemical reactions are reversible, but this reversibility may not be observable if the fraction of products in the equilibrium mixture is very small, or if the reverse reaction is kinetic ...
Reaction Kinetics Basics
... The reaction steps in the mechanism of a homogeneous gas-phase reaction are usually elementary reactions, that is, the stoichiometric equation of the reaction step corresponds to real molecular changes. The molecularity of an elementary reaction is the number of molecular entities involved in the mo ...
... The reaction steps in the mechanism of a homogeneous gas-phase reaction are usually elementary reactions, that is, the stoichiometric equation of the reaction step corresponds to real molecular changes. The molecularity of an elementary reaction is the number of molecular entities involved in the mo ...
Review Package KCI 2017 Sem 1
... a catalyst provides an alternate “pathway”, with lower activation energy, to the same product formation, meaning a much larger fraction of collisions are effective the catalyst can help break the bonds in the reactant particles, provide a surface for the necessary collisions, and allow the react ...
... a catalyst provides an alternate “pathway”, with lower activation energy, to the same product formation, meaning a much larger fraction of collisions are effective the catalyst can help break the bonds in the reactant particles, provide a surface for the necessary collisions, and allow the react ...