Microwave-Specific Enhancement of the Carbon−Carbon Dioxide
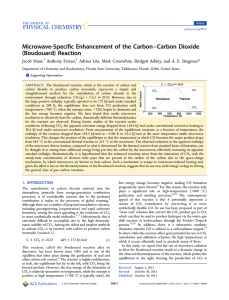
... Figure 2b. As can be seen in Figure 2, product formation is quite linear with time. We do not observe the initial outgassing process that is observed in microwave heating because the carbon is brought up to the desired temperature under an inert atmosphere prior to flowing CO2 over it. The rates of r ...
... Figure 2b. As can be seen in Figure 2, product formation is quite linear with time. We do not observe the initial outgassing process that is observed in microwave heating because the carbon is brought up to the desired temperature under an inert atmosphere prior to flowing CO2 over it. The rates of r ...
CARBANIONS Carbanions are units that contain a negative charge
... changes and formation of new carbon-carbon bonds. Carbanions are very useful intermediates for the formation of new carbon-carbon bonds. Thus carbanions participate in 1) SN2 alkylation reactions, 2) in 1,2 additions to carbonyl functions, and 3) in 1,4-additions such as Michael Reactions. Fluorinat ...
... changes and formation of new carbon-carbon bonds. Carbanions are very useful intermediates for the formation of new carbon-carbon bonds. Thus carbanions participate in 1) SN2 alkylation reactions, 2) in 1,2 additions to carbonyl functions, and 3) in 1,4-additions such as Michael Reactions. Fluorinat ...
Topics 7 and 17 Outlines
... • Physical and chemical systems should be covered. • Relationship between Kc values for reactions that are multiples or inverses of one another should be covered. • Specific details of any industrial process are not required. 17.1 The equilibrium law Essential idea: The position of equilibrium can b ...
... • Physical and chemical systems should be covered. • Relationship between Kc values for reactions that are multiples or inverses of one another should be covered. • Specific details of any industrial process are not required. 17.1 The equilibrium law Essential idea: The position of equilibrium can b ...
Balancing Chemical Reactions
... common mistakes when balancing a chemical reaction is to change the subscripts on compounds instead of changing the stoichiometric coefficients. For example, in the presence of a spark, gaseous mixtures of H2 and O2 react forming water as the only product. The unbalanced reaction based on this descr ...
... common mistakes when balancing a chemical reaction is to change the subscripts on compounds instead of changing the stoichiometric coefficients. For example, in the presence of a spark, gaseous mixtures of H2 and O2 react forming water as the only product. The unbalanced reaction based on this descr ...
Homogeneous Catalysis
... from rate constants at different temperatures. - Derive rate law from reaction mechanism. - The role of catalyst - Homogenous and heterogeneous catalyst ...
... from rate constants at different temperatures. - Derive rate law from reaction mechanism. - The role of catalyst - Homogenous and heterogeneous catalyst ...
PPT
... “Flash Photolysis” – e.g., Bryukov et al., J. Phys. Chem. A., 2004, v. 108, 10464-10472. OH + CH4 H2O + CH3 Basic requirements: A fast (ms to ms) method for detecting and quantifying [OH], as it decays via reaction with CH4. Generally, detection is via absorption or fluorescence-based methodologie ...
... “Flash Photolysis” – e.g., Bryukov et al., J. Phys. Chem. A., 2004, v. 108, 10464-10472. OH + CH4 H2O + CH3 Basic requirements: A fast (ms to ms) method for detecting and quantifying [OH], as it decays via reaction with CH4. Generally, detection is via absorption or fluorescence-based methodologie ...
General Sciences Sample First Exercise Propanoic Acid Solution
... Thus the statement is true. II-2-b) Propanoic acid is a weak acid, so Cacid ≠ [H3O+] then C1 ≠ [H3O+]S1. But pH1 = - log [H3O+]1 then log C1 ≠ pH1. Thus the statement is false. II-2-c) F = 100; for strong acids, upon dilution by 100 folds the pH increases by 2 units. If this acid were strong then pH ...
... Thus the statement is true. II-2-b) Propanoic acid is a weak acid, so Cacid ≠ [H3O+] then C1 ≠ [H3O+]S1. But pH1 = - log [H3O+]1 then log C1 ≠ pH1. Thus the statement is false. II-2-c) F = 100; for strong acids, upon dilution by 100 folds the pH increases by 2 units. If this acid were strong then pH ...























