Chemical reactions
... interaction, in general (not only for a gas, but for condense matter, and even for matter-radiation interaction). Usually the collision is not very energetic, and we say that it is of thermal type (corresponding to mechanical dissipation and heat transfer), but sometimes it is so energetic that mole ...
... interaction, in general (not only for a gas, but for condense matter, and even for matter-radiation interaction). Usually the collision is not very energetic, and we say that it is of thermal type (corresponding to mechanical dissipation and heat transfer), but sometimes it is so energetic that mole ...
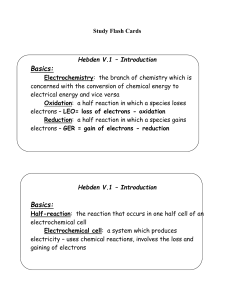
Reduction
... The disproportionation of Cu(I) is the result of combining half-reactions (1) and (3). Thus ...
... The disproportionation of Cu(I) is the result of combining half-reactions (1) and (3). Thus ...
AT 25 °C - University of Bath
... Hence, at 450 °C, 108.24 kJ is evolved for each mole of the equation i.e 108.24 kJ is evolved per mole of nitrogen which reacts. Since, for a gas, volume ∝ number of moles, the 1:3 mixture is in the stoichiometric amount. At 0 °C and 1 atm pressure, 1 mole occupies 22.4 dm3. Thus, the total amount o ...
... Hence, at 450 °C, 108.24 kJ is evolved for each mole of the equation i.e 108.24 kJ is evolved per mole of nitrogen which reacts. Since, for a gas, volume ∝ number of moles, the 1:3 mixture is in the stoichiometric amount. At 0 °C and 1 atm pressure, 1 mole occupies 22.4 dm3. Thus, the total amount o ...
AP Syllabus 95-96 - Bremen High School District 228
... AP courses are college level courses offered in a high school setting. This requires some compromise in the handling of routine matters, such as attendance and makeup work. Stated below are certain policies for this course. Some flexibility may apply in special circumstances and students are encoura ...
... AP courses are college level courses offered in a high school setting. This requires some compromise in the handling of routine matters, such as attendance and makeup work. Stated below are certain policies for this course. Some flexibility may apply in special circumstances and students are encoura ...
Size-Selective Hydrogenation of Olefins by Dendrimer
... open to the atmosphere at the top. All of the hydrogenation reactions were run at atmospheric pressure and room temperature (25 ( 2 °C). Hydrogenation Reactions. 50 mL of the catalytic solution and a magnetic stir bar were placed in a Schlenk flask. All of the joints of the apparatus were sealed wit ...
... open to the atmosphere at the top. All of the hydrogenation reactions were run at atmospheric pressure and room temperature (25 ( 2 °C). Hydrogenation Reactions. 50 mL of the catalytic solution and a magnetic stir bar were placed in a Schlenk flask. All of the joints of the apparatus were sealed wit ...
Answers to Homework Problem Sheet 11
... Between experiments (3) and (4), [Fe2+] and [H+] are unchanged. [O2] is doubled and this doubles the rate: the reaction is first-order with respect to [O2]. Between experiments (1) and (3), [O2] is unchanged. [Fe2+] and [H+] are both doubled and this leads to the rate increasing by a factor of 16. A ...
... Between experiments (3) and (4), [Fe2+] and [H+] are unchanged. [O2] is doubled and this doubles the rate: the reaction is first-order with respect to [O2]. Between experiments (1) and (3), [O2] is unchanged. [Fe2+] and [H+] are both doubled and this leads to the rate increasing by a factor of 16. A ...
Kinetics and Equilibrium ___ 1. In a chemical reaction the use of a
... ___ 16. The equilibrium constant value for a sample of water at 1 atmosphere and 298 °K will be most likely to change when there is an increase in the (1) concentration of H1+ ions; (2) concentration of OH1– ions; (3) pressure; (4) temperature. ___ 17. Which change may occur in a reaction system whe ...
... ___ 16. The equilibrium constant value for a sample of water at 1 atmosphere and 298 °K will be most likely to change when there is an increase in the (1) concentration of H1+ ions; (2) concentration of OH1– ions; (3) pressure; (4) temperature. ___ 17. Which change may occur in a reaction system whe ...
SF Chemical Kinetics Michaelmas 2011 L5
... • The theoretical approach is based on the kinetic theory of gases. • Molecules are assumed to be hard structureless spheres. Hence the model neglects the discrete chemical structure of an individual molecule. This assumption is unrealistic. • We also assume that no interaction between molecules unt ...
... • The theoretical approach is based on the kinetic theory of gases. • Molecules are assumed to be hard structureless spheres. Hence the model neglects the discrete chemical structure of an individual molecule. This assumption is unrealistic. • We also assume that no interaction between molecules unt ...
Unit 1 - Physical Chemistry REACTION KINETICS
... Since step ② is the rate determining step, it is no surprise that it is first order with respect to O2 since one molecule of O2 is involved. The Rate also depends on the concentration of the intermediate, N2O2. However, this, in turn, is determined by how quickly step ① can produce the N2O2 needed i ...
... Since step ② is the rate determining step, it is no surprise that it is first order with respect to O2 since one molecule of O2 is involved. The Rate also depends on the concentration of the intermediate, N2O2. However, this, in turn, is determined by how quickly step ① can produce the N2O2 needed i ...
Hebden V.2 – Oxidation Numbers
... 1. oxidizing agents are on the right 2. reducing agents are on the left 3. oxidizing agents are strongest at the top 4. reducing agents are strongest at the bottom 5. some elements may be oxidizing or reducing agents 6. use double arrows to show the reactions can go forward or reverse when you are t ...
... 1. oxidizing agents are on the right 2. reducing agents are on the left 3. oxidizing agents are strongest at the top 4. reducing agents are strongest at the bottom 5. some elements may be oxidizing or reducing agents 6. use double arrows to show the reactions can go forward or reverse when you are t ...