CHEMICAL KINETICS
... Enantiomers have identical atoms and bonds, but the two different forms have different optical properties. If plane polarized light is passed through a solution of a chiral compound. the plane of polarization of the light is rotated either clockwise or counterclockwise. The extent of this rotation d ...
... Enantiomers have identical atoms and bonds, but the two different forms have different optical properties. If plane polarized light is passed through a solution of a chiral compound. the plane of polarization of the light is rotated either clockwise or counterclockwise. The extent of this rotation d ...
Application of the Purdue Ontology for Pharmaceutical Engineering
... There had been some work done to describe materials in an explicit manner including the Standard for the Exchange of Product Data STEP (ISO 10303) and OntoCAPE, which included descriptions of phases, chemical components and reactions (Yang and Marquardt, 2004). However, in the data models above, exp ...
... There had been some work done to describe materials in an explicit manner including the Standard for the Exchange of Product Data STEP (ISO 10303) and OntoCAPE, which included descriptions of phases, chemical components and reactions (Yang and Marquardt, 2004). However, in the data models above, exp ...
review CH5 chem121pikul
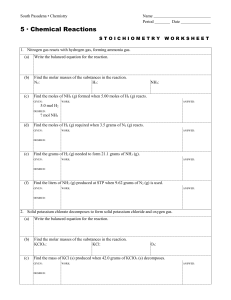
... ! Define a chemical reaction ! Correctly write a chemical reaction ! Balance reactions by inspection ! Calculate molecular mass for any compound or molecule ! Apply mole ratios within molecules and between molecules. ! Solve stoichiometry problems ! Convert between mass and moles ! Convert b ...
... ! Define a chemical reaction ! Correctly write a chemical reaction ! Balance reactions by inspection ! Calculate molecular mass for any compound or molecule ! Apply mole ratios within molecules and between molecules. ! Solve stoichiometry problems ! Convert between mass and moles ! Convert b ...
Examination 1 - Idaho State University
... stoichiometrically until Qc = Ksp. You should be able to decide if, what ppt., and how much ppt. will form if solution which is a mixture of salts. Of course you will need to know the solubility rules to do this. How does adding an acid affect the solubility of certain insoluble ionic compounds? Wha ...
... stoichiometrically until Qc = Ksp. You should be able to decide if, what ppt., and how much ppt. will form if solution which is a mixture of salts. Of course you will need to know the solubility rules to do this. How does adding an acid affect the solubility of certain insoluble ionic compounds? Wha ...
Course Home - Haldia Institute of Technology
... FT301.1 Ability to understand the basics and meanings of thermodynamics and kinetics. FT301.2 Ability to understand and introduce the laws of thermodynamics, their implications, and become familiar with their use and applications. FT301.3 Ability to predict intermolecular potential and excess proper ...
... FT301.1 Ability to understand the basics and meanings of thermodynamics and kinetics. FT301.2 Ability to understand and introduce the laws of thermodynamics, their implications, and become familiar with their use and applications. FT301.3 Ability to predict intermolecular potential and excess proper ...