Cellular Respiration
... - The ATP produced is actually GTP, or guanosine triphosphate, a similar, energy-rich molecule that can also be used to do work (but eh same thing) - ATP is generated through substrate-level phosphorylation with the help of kinases once again - Both NADH and FADH2 are electron carriers that transp ...
... - The ATP produced is actually GTP, or guanosine triphosphate, a similar, energy-rich molecule that can also be used to do work (but eh same thing) - ATP is generated through substrate-level phosphorylation with the help of kinases once again - Both NADH and FADH2 are electron carriers that transp ...
Chapter 8 Learning Targets(141- 150)
... 2. I can explain how glycolysis harvests chemical energy by oxidizing glucose to pyruvate. a. I can list the reactants and products of glycolysis, and describe how the carbon skeleton of glucose changes during this process. b. I can specifically describe where glycolysis is occurring in a cell. c. I ...
... 2. I can explain how glycolysis harvests chemical energy by oxidizing glucose to pyruvate. a. I can list the reactants and products of glycolysis, and describe how the carbon skeleton of glucose changes during this process. b. I can specifically describe where glycolysis is occurring in a cell. c. I ...
BIO 101 Exam 2 practice questions Practice questions Ch 8,9 YOU
... 31. ATP synthase can produce ATP using as a direct energy source: a. energy from the conversion of glucose to pyruvate b. energy from the oxidation of pyruvate producing CO2 and H20 c. energy from a proton (H+) gradient established in mitochondria d. energy derived from the breakdown of NADH and FAD ...
... 31. ATP synthase can produce ATP using as a direct energy source: a. energy from the conversion of glucose to pyruvate b. energy from the oxidation of pyruvate producing CO2 and H20 c. energy from a proton (H+) gradient established in mitochondria d. energy derived from the breakdown of NADH and FAD ...
Cellular Respiration
... Small amounts of energy are released in each step Electrons eventually reach Oxygen (remember Oxygen is one of the most electronegative elements!) ...
... Small amounts of energy are released in each step Electrons eventually reach Oxygen (remember Oxygen is one of the most electronegative elements!) ...
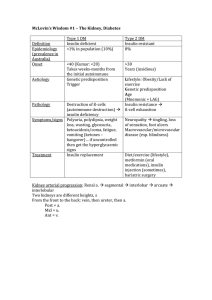
McLovin`s Wisdom #1 – The Kidney, Diabetes Type 1 DM Type 2
... At complex 4, 1/2O2 + 2H+ H2O (the H+s are reacted with oxygen to reduce it to water. Hence oxygen is needed). ATP synthase. 4H+ going through ATP synthase produce 1 ATP (3H+ go through there, and 1H+ used to transport the ATP back out into the intermembrane space – the outer mitochondrial membra ...
... At complex 4, 1/2O2 + 2H+ H2O (the H+s are reacted with oxygen to reduce it to water. Hence oxygen is needed). ATP synthase. 4H+ going through ATP synthase produce 1 ATP (3H+ go through there, and 1H+ used to transport the ATP back out into the intermembrane space – the outer mitochondrial membra ...
скачати - ua
... Glycolysis, the Universal Process | Nine reactions, each catalyzed by a specific enzyme, makeup the process we call glycolysis. ALL organisms have glycolysis occurring in their cytoplasm. At steps 1 and 3 ATP is converted into ADP, inputting energy into the reaction as well as attaching a phosphate ...
... Glycolysis, the Universal Process | Nine reactions, each catalyzed by a specific enzyme, makeup the process we call glycolysis. ALL organisms have glycolysis occurring in their cytoplasm. At steps 1 and 3 ATP is converted into ADP, inputting energy into the reaction as well as attaching a phosphate ...
cellular respiration
... through the process of cellular respiration. Cells take the carbohydrates into their cytoplasm, and through a complex series of metabolic processes, they break down the carbohydrates and release the energy. The energy is generally not needed immediately; rather it is used to combine adenosine diphos ...
... through the process of cellular respiration. Cells take the carbohydrates into their cytoplasm, and through a complex series of metabolic processes, they break down the carbohydrates and release the energy. The energy is generally not needed immediately; rather it is used to combine adenosine diphos ...
Use of Reduced Carbon Compounds
... --- the point of the Calvin cycle is to “fix” carbon, create reduced carbon compounds that can be used for biosynthesis or stored for later conversion into cellular energy CO2 CH2OH --- this process requires tremendous amounts of energy, 3 ATP and 2 NADPH per CH2OH unit (18 ATP and 12 NADPH per 6 ...
... --- the point of the Calvin cycle is to “fix” carbon, create reduced carbon compounds that can be used for biosynthesis or stored for later conversion into cellular energy CO2 CH2OH --- this process requires tremendous amounts of energy, 3 ATP and 2 NADPH per CH2OH unit (18 ATP and 12 NADPH per 6 ...
BY 123 Mock Exam #2 Answer Key Chapters 8,9,10,12,13 Catabolic
... Some prokaryotes use anaerobic respiration, a process that: a. Does not involve an electron transport chain b. Produces ATP solely by substrate-level phosphorylation c. Uses a substance other than oxygen as the final electron acceptor d. Does not rely on chemiosmosis for the production of ATP e. Bot ...
... Some prokaryotes use anaerobic respiration, a process that: a. Does not involve an electron transport chain b. Produces ATP solely by substrate-level phosphorylation c. Uses a substance other than oxygen as the final electron acceptor d. Does not rely on chemiosmosis for the production of ATP e. Bot ...
AMPK and mTOR: Antagonist ATP Sensors
... of ATP, referred to as a “high-energy phosphate”, is made up of adenine and ribose (adenosine) bonded to three phosphates (Pi- phosphorus and oxygen). The energy stored in ATP is held in the two outermost phosphate bonds. These outermost bonds are referred to as “high-energy bonds.” When water joins ...
... of ATP, referred to as a “high-energy phosphate”, is made up of adenine and ribose (adenosine) bonded to three phosphates (Pi- phosphorus and oxygen). The energy stored in ATP is held in the two outermost phosphate bonds. These outermost bonds are referred to as “high-energy bonds.” When water joins ...
Intro to Metabolism
... d. The bond between the alpha phosphate and the ribose is an ester bond, which is another dehydration reaction between an acid and an alcohol. e. High-energy bond, in this case, refers not to the energy of the bond, but the energy that is released during transfer reactions. f. Here is a simple examp ...
... d. The bond between the alpha phosphate and the ribose is an ester bond, which is another dehydration reaction between an acid and an alcohol. e. High-energy bond, in this case, refers not to the energy of the bond, but the energy that is released during transfer reactions. f. Here is a simple examp ...
Metabolism
... Antioxidants – compounds that donate electrons to oxidized compounds, putting them into a more reduced (stable) state Oxidized compounds tend to be highly reactive Vitamins E and C are antioxidants Remember phytochemicals! ...
... Antioxidants – compounds that donate electrons to oxidized compounds, putting them into a more reduced (stable) state Oxidized compounds tend to be highly reactive Vitamins E and C are antioxidants Remember phytochemicals! ...
Introduction to: Cellular Respiration
... The reason we eat is to get energy We get carbohydrates from our food which are broken into Glucose Organisms cannot use glucose directly, it must be broken down into smaller units…… ATP This process in living things begins with glycolysis. If oxygen is present, glycolysis is followed by the K ...
... The reason we eat is to get energy We get carbohydrates from our food which are broken into Glucose Organisms cannot use glucose directly, it must be broken down into smaller units…… ATP This process in living things begins with glycolysis. If oxygen is present, glycolysis is followed by the K ...
Unit7CellRespirationTargetPractice
... concentration of protons is _________________ in the intermembrane space than in the matrix of the mitochondria. The protons cannot freely _____________ across the inner membrane of the mitochondria. Protons move across the inner membrane via a large protein called _________________; the energy rele ...
... concentration of protons is _________________ in the intermembrane space than in the matrix of the mitochondria. The protons cannot freely _____________ across the inner membrane of the mitochondria. Protons move across the inner membrane via a large protein called _________________; the energy rele ...
the Four Stages of Biochemical Energy Production
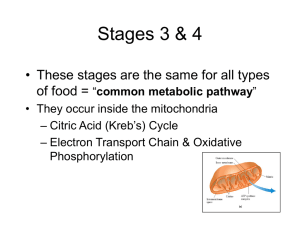
... Citric acid cycle – For every glucose, two acetyl groups enter the citric acid cycle (Krebs cycle) • Each two-carbon acetyl group combines with a fourcarbon compound • Two CO2 molecules are removed (why is this important?) • Energy captured as 1 ATP, 3 NADH, and 1 FADH2 form from each acetyl group ...
... Citric acid cycle – For every glucose, two acetyl groups enter the citric acid cycle (Krebs cycle) • Each two-carbon acetyl group combines with a fourcarbon compound • Two CO2 molecules are removed (why is this important?) • Energy captured as 1 ATP, 3 NADH, and 1 FADH2 form from each acetyl group ...
Biology
... 6. What are the four chemicals that are exchanged over and over again in the cycles of photosynthesis and cellular respiration? Draw a diagram that summarizes the relationship between the two processes and label it with these four chemicals, in addition to the light energy required and the chemical ...
... 6. What are the four chemicals that are exchanged over and over again in the cycles of photosynthesis and cellular respiration? Draw a diagram that summarizes the relationship between the two processes and label it with these four chemicals, in addition to the light energy required and the chemical ...
Biochemistry Study Guide – Exam 1
... Energy, entropy, enthalpy Equations for enthalpy change Free energy and free energy changes ...
... Energy, entropy, enthalpy Equations for enthalpy change Free energy and free energy changes ...
HW #23 KEY 1. Adenosine triphosphate is the energy currency of
... 41. The end products of cellular respiration are CO2 and H2O. Where do the oxygen atoms in the CO2 originate? Where does the oxygen atom in H2O originate? The oxygen atoms in carbon dioxide come from glucose, and the oxygen in water comes from the atmosphere. 42. What is the advantage of aerobic met ...
... 41. The end products of cellular respiration are CO2 and H2O. Where do the oxygen atoms in the CO2 originate? Where does the oxygen atom in H2O originate? The oxygen atoms in carbon dioxide come from glucose, and the oxygen in water comes from the atmosphere. 42. What is the advantage of aerobic met ...
Chapters 13 and 16
... precursors in amino acid and nucleotide synthesis (example, α-ketoglutarate can be converted into glutamate and then to other amino acids). Oxaloacetate can be converted back to glucose. In addition, there are reactions to supply some of the TCA intermediates (Glu can be converted back to α-ketoglut ...
... precursors in amino acid and nucleotide synthesis (example, α-ketoglutarate can be converted into glutamate and then to other amino acids). Oxaloacetate can be converted back to glucose. In addition, there are reactions to supply some of the TCA intermediates (Glu can be converted back to α-ketoglut ...
Chapter 9: Cellular Respiration and Fermentation
... and into the intermembrane space creating a Conc. Gradient The only way back into the matrix for H+ ions is through a protein called ATP Synthase. As H+ move through ATP Synthase like water through a dam, energy is used to convert ADP to ATP. Each pair of electrons can produce between two and three ...
... and into the intermembrane space creating a Conc. Gradient The only way back into the matrix for H+ ions is through a protein called ATP Synthase. As H+ move through ATP Synthase like water through a dam, energy is used to convert ADP to ATP. Each pair of electrons can produce between two and three ...
Document
... • How did we get from glucose to lactic acid? • In the liver, the process is “reversed” using ATP from aerobic respiration ...
... • How did we get from glucose to lactic acid? • In the liver, the process is “reversed” using ATP from aerobic respiration ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.