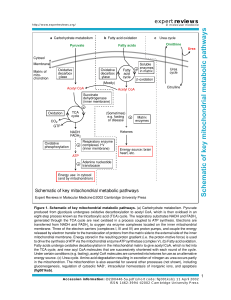
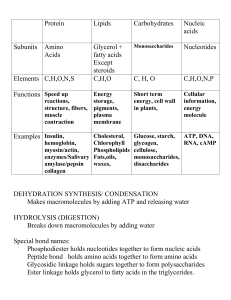
CellularRespirationReview
... Photosynthesis gets electrons from— breakdown of water molecules (photolysis). Cellular respiration gets electrons from— FADH2 and NADH from Krebs cycle. Final electron acceptors= NADP+ in photosynthesis and Oxygen in aerobic respiration. ...
... Photosynthesis gets electrons from— breakdown of water molecules (photolysis). Cellular respiration gets electrons from— FADH2 and NADH from Krebs cycle. Final electron acceptors= NADP+ in photosynthesis and Oxygen in aerobic respiration. ...
Energy Production
... starts to accumulate lowering the pH to a level that interferes with enzymatic reaction causing fatigue. * Anaerobic pathway provides energy for short duration, high intensity exercise such as sprinting and high intensity moves in basketball or in football. ...
... starts to accumulate lowering the pH to a level that interferes with enzymatic reaction causing fatigue. * Anaerobic pathway provides energy for short duration, high intensity exercise such as sprinting and high intensity moves in basketball or in football. ...
Metabolic Adaptation - Washington State University
... • Epinephrine released in cold stress or spring rewarming causes production of uncoupling factors that induce a proton leak in inner mitochondrial membrane. Consequently, all of the free energy released by oxidative metabolism of fat appears as heat. ...
... • Epinephrine released in cold stress or spring rewarming causes production of uncoupling factors that induce a proton leak in inner mitochondrial membrane. Consequently, all of the free energy released by oxidative metabolism of fat appears as heat. ...
Harvesting Energy: Glycolysis and Cellular Respiration Using the
... •The cell produces only 4 ATP molecules during glycolysis and the Krebs cycle. •Additional high-energy electron carriers have been made (10 NADH and 2 FADH2). •Along the inner membrane of the mitochondria, ...
... •The cell produces only 4 ATP molecules during glycolysis and the Krebs cycle. •Additional high-energy electron carriers have been made (10 NADH and 2 FADH2). •Along the inner membrane of the mitochondria, ...
METABOLISM - Doctor Jade Main
... channels allow H ions to enter matrix Chemiosmosis – energy released during oxidation of fuels=chemi – pumping H ions across membranes of mitochondria into inter membrane space =osmo – creates steep diffusion gradient for Hs across membrane when hydrogens flow across membrane, through membrane chann ...
... channels allow H ions to enter matrix Chemiosmosis – energy released during oxidation of fuels=chemi – pumping H ions across membranes of mitochondria into inter membrane space =osmo – creates steep diffusion gradient for Hs across membrane when hydrogens flow across membrane, through membrane chann ...
Bio 201, Fall 2010 Test 3 Study Guide Questions to be able to
... 9. How are desmosomes different from tight junctions? 10. Differentiate between plasma membrane use in plasmosdesmata and gap junctions. 11. Where are gap junctions found, and why is it important that calcium and cAMP are small enough to pass through gap junctions? 12. What is the purpose of chemica ...
... 9. How are desmosomes different from tight junctions? 10. Differentiate between plasma membrane use in plasmosdesmata and gap junctions. 11. Where are gap junctions found, and why is it important that calcium and cAMP are small enough to pass through gap junctions? 12. What is the purpose of chemica ...
Cellular Respiration Chapter 9
... If no oxygen is present after glycolysis, what process occurs? Is this a more efficient pathway? ...
... If no oxygen is present after glycolysis, what process occurs? Is this a more efficient pathway? ...
Photosynthesis & Respiration
... • Net total of 36 ATP produced • Formula C6H12O6 + 6O2 glucose + oxygen ...
... • Net total of 36 ATP produced • Formula C6H12O6 + 6O2 glucose + oxygen ...
Chapter 4: Cellular Metabolism
... __________________________________________________________________ 2. The genetic code is________________________________________________ B. Genetic Information 1. Cell structures that carry DNA are____________________________________ 2. A gene is ____________________________________________________ ...
... __________________________________________________________________ 2. The genetic code is________________________________________________ B. Genetic Information 1. Cell structures that carry DNA are____________________________________ 2. A gene is ____________________________________________________ ...
September 17 Worksheet Answer Key
... 6. Briefly describe Patrick’s case study. What was wrong with Patrick? Patrick had a genetic condition such that he had a deficiency of PDH. 7. TEST QUESTION: Which of the following is not true regarding energy? a. Potential energy represents stored energy; eg. energy in chemical bonds. b. Kinetic e ...
... 6. Briefly describe Patrick’s case study. What was wrong with Patrick? Patrick had a genetic condition such that he had a deficiency of PDH. 7. TEST QUESTION: Which of the following is not true regarding energy? a. Potential energy represents stored energy; eg. energy in chemical bonds. b. Kinetic e ...
CELLULAR RESPIRATION
... mitochondria. Because eukaryotic cells have mitochondria, the citric acid cycle and electron transport chain __________________ ADVANCEMENTS that result in more efficient ATP production in this organelle type. • Because in cytoplasma, may be why O2 not required ...
... mitochondria. Because eukaryotic cells have mitochondria, the citric acid cycle and electron transport chain __________________ ADVANCEMENTS that result in more efficient ATP production in this organelle type. • Because in cytoplasma, may be why O2 not required ...
bio II ch 8 brookings guided pp
... = using energy from breaking a chemical bond to add MITOCHONDRION aP directly from a phosphorylated molecule to ADP without a proton gradient ...
... = using energy from breaking a chemical bond to add MITOCHONDRION aP directly from a phosphorylated molecule to ADP without a proton gradient ...
Bio102 Problems
... 17. We can isolate mitochondria from liver cells and measure the rate at which electron transport proceeds as well as their ability to make ATP. Then we can modify their phospholipids and observe the following effects: 17A. If we modify the phospholipids in the mitochondria so that they are less uns ...
... 17. We can isolate mitochondria from liver cells and measure the rate at which electron transport proceeds as well as their ability to make ATP. Then we can modify their phospholipids and observe the following effects: 17A. If we modify the phospholipids in the mitochondria so that they are less uns ...
Oxidations – loss of electrons
... – Use of inorganic molecules (other than O2) as final electron acceptor – Many prokaryotes use sulfur, nitrate, carbon dioxide or even inorganic metals ...
... – Use of inorganic molecules (other than O2) as final electron acceptor – Many prokaryotes use sulfur, nitrate, carbon dioxide or even inorganic metals ...
Cellular Respiration - Mrs. Brenner`s Biology
... Lactic acid vs. Alcoholic Fermentation • Yeasts + other organisms • pyruvic acid +NADH alcohol + CO2 + NAD ...
... Lactic acid vs. Alcoholic Fermentation • Yeasts + other organisms • pyruvic acid +NADH alcohol + CO2 + NAD ...
`Metabolic flux` describes the rate of flow of intermediates through a
... Negative effector (non-biological); stabilizes T-state ...
... Negative effector (non-biological); stabilizes T-state ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.