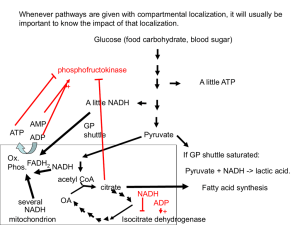
Cellular Respiration Notes (Overhead Version)
... The ELECTRON TRANSPORT CHAIN makes up the Second Stage of Aerobic Respiration. It LINES the INNER MEMBRANE of the Mitochondrion, the inner membrane has many long folds called CRISTAE. ATP is produced by the Electron Transport Chain when NADH and FADH2 RELEASES Hydrogen Atoms, REGENERATING NAD+ and ...
... The ELECTRON TRANSPORT CHAIN makes up the Second Stage of Aerobic Respiration. It LINES the INNER MEMBRANE of the Mitochondrion, the inner membrane has many long folds called CRISTAE. ATP is produced by the Electron Transport Chain when NADH and FADH2 RELEASES Hydrogen Atoms, REGENERATING NAD+ and ...
Practice exam #1 review
... b. NAPH and FAPH2 c. ATP d. H2O e. all the above Modified True or False Write T or F at each question and if false correct then make it true. 1. ATP is an energy intermediate T F 2. ATP releases energy when the bond undergoes a dehydration reaction T F 3. Delta G is negative when the products have l ...
... b. NAPH and FAPH2 c. ATP d. H2O e. all the above Modified True or False Write T or F at each question and if false correct then make it true. 1. ATP is an energy intermediate T F 2. ATP releases energy when the bond undergoes a dehydration reaction T F 3. Delta G is negative when the products have l ...
Cell Respiration Basics
... During the Krebs cycle Acetyl CoA molecules formed from pyruvic acid molecules, are broken down. CO2 is given off, and ATP is produced. (1 ATP per each pyruvic acid or each turn of the cycle.) ...
... During the Krebs cycle Acetyl CoA molecules formed from pyruvic acid molecules, are broken down. CO2 is given off, and ATP is produced. (1 ATP per each pyruvic acid or each turn of the cycle.) ...
Energy Metabolism - Georgia Institute of Technology
... – H+ actively transported out of matrix – H+ leak back as H+PO4 2- ...
... – H+ actively transported out of matrix – H+ leak back as H+PO4 2- ...
Chapter 9 outline
... During oxidative phosphorylation, chemiosmosis couples electron transport to ATP synthesis NADH and FADH2 – Donate electrons to the electron transport chain, which powers ATP synthesis via oxidative phosphorylation ...
... During oxidative phosphorylation, chemiosmosis couples electron transport to ATP synthesis NADH and FADH2 – Donate electrons to the electron transport chain, which powers ATP synthesis via oxidative phosphorylation ...
Glycolysis and fermentation
... Glucose is broken down with or without oxygen in the cytoplasm into pyruvate One Glucose is cleaved into two pyruvate Produces little energy Two ATP and Two NADH produced ...
... Glucose is broken down with or without oxygen in the cytoplasm into pyruvate One Glucose is cleaved into two pyruvate Produces little energy Two ATP and Two NADH produced ...
Document
... Glucose is broken down with or without oxygen in the cytoplasm into pyruvate One Glucose is cleaved into two pyruvate Produces little energy Two ATP and Two NADH produced ...
... Glucose is broken down with or without oxygen in the cytoplasm into pyruvate One Glucose is cleaved into two pyruvate Produces little energy Two ATP and Two NADH produced ...
Respiration
... Ø it is a valuable source of intermediates which are used to manufacture other substances e.g. fatty acid, amino acids etc. (c) Electron transport chain / oxidative phosphorylation : : the hydrogen atoms produced in glycolysis and Krebs cycle are carried by NAD or FAD as NADH 2 and FADH 2 respective ...
... Ø it is a valuable source of intermediates which are used to manufacture other substances e.g. fatty acid, amino acids etc. (c) Electron transport chain / oxidative phosphorylation : : the hydrogen atoms produced in glycolysis and Krebs cycle are carried by NAD or FAD as NADH 2 and FADH 2 respective ...
lecture 02b
... which needs to be “cashed in” to make ATP. – In order for glycolysis and Krebs Cycle to continue, NAD that gets reduced to NADH must get re-oxidized to NAD. – What is the greediest electron hog we know? Molecular oxygen. – In Electron transport, electrons are passed to oxygen so that these metabolic ...
... which needs to be “cashed in” to make ATP. – In order for glycolysis and Krebs Cycle to continue, NAD that gets reduced to NADH must get re-oxidized to NAD. – What is the greediest electron hog we know? Molecular oxygen. – In Electron transport, electrons are passed to oxygen so that these metabolic ...
Cellular Biology I
... Valence shell = the outermost energy level that has any electrons in it Atoms are most stable when the valence shell is filled – see noble gases of periodic table Some atoms can “steal” electrons to fill valences shell; they are “ionized” – no longer neutral – Ex. ...
... Valence shell = the outermost energy level that has any electrons in it Atoms are most stable when the valence shell is filled – see noble gases of periodic table Some atoms can “steal” electrons to fill valences shell; they are “ionized” – no longer neutral – Ex. ...
Agrobacterium tumefaciens
... Eg. 2 Beetals may have very low level of fat soluble pesticides, but the pesticides will build much greater levels till in a human that eats the beetal eating bird. Eg. 3 DDT in food chain of coastal water of long island. ...
... Eg. 2 Beetals may have very low level of fat soluble pesticides, but the pesticides will build much greater levels till in a human that eats the beetal eating bird. Eg. 3 DDT in food chain of coastal water of long island. ...
Cellular respiration *vs
... So—can energy be produced without oxygen? YES!! It is called “Fermentation.” • Sometimes we do not get as much oxygen to our cells as they prefer. If you remember the word anaerobic—means w/o oxygen--fermentation is a form of anaerobic respiration. • Because there is no oxygen for use the ATP for e ...
... So—can energy be produced without oxygen? YES!! It is called “Fermentation.” • Sometimes we do not get as much oxygen to our cells as they prefer. If you remember the word anaerobic—means w/o oxygen--fermentation is a form of anaerobic respiration. • Because there is no oxygen for use the ATP for e ...
File
... c. What are the roles of NAD+ & FAD+2 in respiration? ___________________________________________ d. Why would AMP stimulate cellular respiration and ATP inhibit it? _____________________________________________________________________________________ 18. STAGE 3: The Electron Transport Chain a. occ ...
... c. What are the roles of NAD+ & FAD+2 in respiration? ___________________________________________ d. Why would AMP stimulate cellular respiration and ATP inhibit it? _____________________________________________________________________________________ 18. STAGE 3: The Electron Transport Chain a. occ ...
Quiz SBI 4UI - Waterloo Region District School Board
... 22. What does the NAD Dehy, Cyt b-c1 and Cyt oxidase have in common? ...
... 22. What does the NAD Dehy, Cyt b-c1 and Cyt oxidase have in common? ...
Energy - My CCSD
... mitochondrial matrix Starts with pyruvate molecules from glycolysis Produces ATP, ecarriers, & some CO2 molecules (CO2 is released from the cell). ...
... mitochondrial matrix Starts with pyruvate molecules from glycolysis Produces ATP, ecarriers, & some CO2 molecules (CO2 is released from the cell). ...
File - Wk 1-2
... 3. Outline the citric acid cycle, listing the main substrates and products of the cycle and the role of the cycle in providing reducing equivalents for the electron transport chain. The citric acid cycle (Krebs cycle) occurs in the mitacholdria of the cell and occurs in the presence of oxygen (aero ...
... 3. Outline the citric acid cycle, listing the main substrates and products of the cycle and the role of the cycle in providing reducing equivalents for the electron transport chain. The citric acid cycle (Krebs cycle) occurs in the mitacholdria of the cell and occurs in the presence of oxygen (aero ...
Cellular Respiration
... each Acetyl CoA (2-C’s) to release 2 CO2 and yield electrons and H+ ions to 3 NAD+ + 1 FAD → 3 NADH + FADH2. This yields energy to produce ATP by substrate level phosphorylation. The first step of the Krebs cycle combines Oxaloacetate (4 C’s) with Acetyl CoA to form Citric Acid, then the remaining 7 ...
... each Acetyl CoA (2-C’s) to release 2 CO2 and yield electrons and H+ ions to 3 NAD+ + 1 FAD → 3 NADH + FADH2. This yields energy to produce ATP by substrate level phosphorylation. The first step of the Krebs cycle combines Oxaloacetate (4 C’s) with Acetyl CoA to form Citric Acid, then the remaining 7 ...
Life History Traits and Genome Structure: Aerobiosis and G+C
... Aerobic versus Anaerobic • You need the presence of O2 to live, you are aerobic (obligate) • You need the absence of O2 to live, you are anaerobic (obligate) • [...snip...] ...
... Aerobic versus Anaerobic • You need the presence of O2 to live, you are aerobic (obligate) • You need the absence of O2 to live, you are anaerobic (obligate) • [...snip...] ...
Lecture 4 - Muscle Metabolism
... • Produces 95% of ATP during rest and light to moderate exercise; slow • Series of chemical reactions that require oxygen; occur in mitochondria – Breaks glucose into CO2, H2O, and large amount ATP ...
... • Produces 95% of ATP during rest and light to moderate exercise; slow • Series of chemical reactions that require oxygen; occur in mitochondria – Breaks glucose into CO2, H2O, and large amount ATP ...
Chapter 5 Microbial Nutrition and Culture
... • Carrier molecules such as Cytochrome (cyt) and some coenzymes carry energy in the form of electrons in many biochemical reactions • Coenzymes such as FAD carry whole hydrogen atoms (electrons together with protons); NAD carries one hydrogen atom and one “naked” electron • When co-enzymes are reduc ...
... • Carrier molecules such as Cytochrome (cyt) and some coenzymes carry energy in the form of electrons in many biochemical reactions • Coenzymes such as FAD carry whole hydrogen atoms (electrons together with protons); NAD carries one hydrogen atom and one “naked” electron • When co-enzymes are reduc ...
Slide 1
... Liver cells have a responsibility to support blood glucose levels by first releasing glucose from their internal glycogen stores, and if necessary synthesizing glucose from amino acids. They will shut down glycolysis and rely on other energy sources for their own needs under these conditions. Liver ...
... Liver cells have a responsibility to support blood glucose levels by first releasing glucose from their internal glycogen stores, and if necessary synthesizing glucose from amino acids. They will shut down glycolysis and rely on other energy sources for their own needs under these conditions. Liver ...
1. Metabolic pathways 2. Basic enzyme kinetics 3. Metabolic
... » Generate energy (ATP) for cellular functions » Produce reducing power (NAPDH) for biosynthesis ...
... » Generate energy (ATP) for cellular functions » Produce reducing power (NAPDH) for biosynthesis ...
Cell Respiration Teacher Notes
... • Glycolysis, Transition reaction, Citric acid cycle (Kreb’s cycle), and Electron transport system • An aerobic process that requires O2 • If oxygen is not available (anaerobic), glycolysis is ...
... • Glycolysis, Transition reaction, Citric acid cycle (Kreb’s cycle), and Electron transport system • An aerobic process that requires O2 • If oxygen is not available (anaerobic), glycolysis is ...
Exam 2 Answers
... a. Generates reducing power in the form of NADH and FADH2 Krebs Cycle b. Produces ATP Glycolysis, Krebs Cycle, ETC c. Begins and ends with a 4-carbon molecule Krebs Cycle d. A catabolic pathway that breaks down glucose to pyruvate in the cytoplasm. Glycolysis e. The means by which the three carbons ...
... a. Generates reducing power in the form of NADH and FADH2 Krebs Cycle b. Produces ATP Glycolysis, Krebs Cycle, ETC c. Begins and ends with a 4-carbon molecule Krebs Cycle d. A catabolic pathway that breaks down glucose to pyruvate in the cytoplasm. Glycolysis e. The means by which the three carbons ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.