03.03a Atomic Number, Mass Number, and Isotopes
... ATOMS: All atoms of the same element have the same number of protons: the number of protons determines the identity of the atom. For example, a carbon atom always has six protons. If it has seven protons, it’s nitrogen, not carbon. The number of protons is called the atomic number (Z). ISOTOPES: Alt ...
... ATOMS: All atoms of the same element have the same number of protons: the number of protons determines the identity of the atom. For example, a carbon atom always has six protons. If it has seven protons, it’s nitrogen, not carbon. The number of protons is called the atomic number (Z). ISOTOPES: Alt ...
Atomic Structure Worksheet
... The Law of Multiple Proportions - Atoms of two or more elements may combine in different ratios to produce more than one compound. • The formula for water is H2O, the formula for hydrogen peroxide is H2O2. • Hydrogen peroxide has twice as many oxygens per hydrogen atom as does water. mass of oxygen ...
... The Law of Multiple Proportions - Atoms of two or more elements may combine in different ratios to produce more than one compound. • The formula for water is H2O, the formula for hydrogen peroxide is H2O2. • Hydrogen peroxide has twice as many oxygens per hydrogen atom as does water. mass of oxygen ...
Practice Test #2 - smhs
... for Ag as 107.868 amu. Find the percent abundance of the lighter isotope of Ag. 107.868 = 106.9041 (X) + 108.9047 (1.000 - X) -1.0367 = -2.0006 X X = 0.51819 ...
... for Ag as 107.868 amu. Find the percent abundance of the lighter isotope of Ag. 107.868 = 106.9041 (X) + 108.9047 (1.000 - X) -1.0367 = -2.0006 X X = 0.51819 ...
Geologic Dating! - rgreenbergscience
... lowest layer is the oldest, the top most layer, the most recent (youngest). These are called “strata”. • Stratification: enables a scientist to establish the “relative age” of something based on where it is found compared to other layers. • Relative Dating – estimating the age of a fossil by compari ...
... lowest layer is the oldest, the top most layer, the most recent (youngest). These are called “strata”. • Stratification: enables a scientist to establish the “relative age” of something based on where it is found compared to other layers. • Relative Dating – estimating the age of a fossil by compari ...
Chemistry lecture notes
... Isotopes have the same atomic number (same number of protons), but a different atomic mass number (a different number of neutrons). Isotopes behave the same chemically, because they are the same element. The only difference is that one is heavier than the other, because of the additional neutron ...
... Isotopes have the same atomic number (same number of protons), but a different atomic mass number (a different number of neutrons). Isotopes behave the same chemically, because they are the same element. The only difference is that one is heavier than the other, because of the additional neutron ...
Chem Unit2 template - Region 7 Professional Development
... Summarizing main points after reading Locating and choosing appropriate reference materials ...
... Summarizing main points after reading Locating and choosing appropriate reference materials ...
Practice Test #2 - smhs
... Answer the following questions based on the -2 anion of an isotopic form of sulfur: S-35 24.________ What is the A number for this nuclide? 25.________ What is the Z number for this nuclide? 26.________ What is the number of protons in the anion form of this nonmetal? 27.________ What is the number ...
... Answer the following questions based on the -2 anion of an isotopic form of sulfur: S-35 24.________ What is the A number for this nuclide? 25.________ What is the Z number for this nuclide? 26.________ What is the number of protons in the anion form of this nonmetal? 27.________ What is the number ...
Unit 2 Test Review - Liberty High School
... to answer these questions on a separate piece of paper (unless you can write microscopically). In addition to these problems, review your notes, assignments (#29-44), labs, and chapter 3 & 4 in your textbook. 1. Classify each as an element (E), a compound (C), or a mixture (M). a. Brass b. Tungste ...
... to answer these questions on a separate piece of paper (unless you can write microscopically). In addition to these problems, review your notes, assignments (#29-44), labs, and chapter 3 & 4 in your textbook. 1. Classify each as an element (E), a compound (C), or a mixture (M). a. Brass b. Tungste ...
File - Norris Science
... the tiny alpha particles would pass through the gold atoms and fly straight into the screen. ...
... the tiny alpha particles would pass through the gold atoms and fly straight into the screen. ...
Chapter 5
... Unlike charges attract and like charges repel Charges may be transferred by contact or induction The closer two object are, the greater the force of attraction ...
... Unlike charges attract and like charges repel Charges may be transferred by contact or induction The closer two object are, the greater the force of attraction ...
Radioisotopes
... having a different atomic mass (mass number) • Isotopes of an element have nuclei with the same number of protons (the same atomic number) but different numbers of neutrons. • Therefore, isotopes have different mass numbers, which give the total number of nucleons, the number of protons plus neutron ...
... having a different atomic mass (mass number) • Isotopes of an element have nuclei with the same number of protons (the same atomic number) but different numbers of neutrons. • Therefore, isotopes have different mass numbers, which give the total number of nucleons, the number of protons plus neutron ...
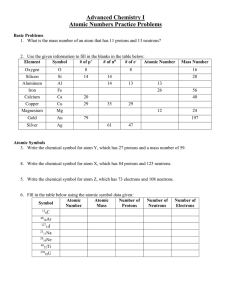
Atomic Numbers Practice Problems
... 3. Write the chemical symbol for atom Y, which has 27 protons and a mass number of 59. ...
... 3. Write the chemical symbol for atom Y, which has 27 protons and a mass number of 59. ...
Hydrogen Models 1
... Atoms & Isotopes Models Purpose: To build 3-D models of the hydrogen atom. Background Information: An atom is defined as a small particle that makes up most types of matter. Atoms are so small it would take about 1 million of them lined up in a row to equal the thickness of a human hair. Atoms are m ...
... Atoms & Isotopes Models Purpose: To build 3-D models of the hydrogen atom. Background Information: An atom is defined as a small particle that makes up most types of matter. Atoms are so small it would take about 1 million of them lined up in a row to equal the thickness of a human hair. Atoms are m ...
Chemical laboratories Dipl.-Ing.(FH) Giovanna
... Sugar and lactic acid analysis by HPLC Ultimate 3000 from Dionex ...
... Sugar and lactic acid analysis by HPLC Ultimate 3000 from Dionex ...
What is an isotope?
... What is an isotope? Number of protons for an atom of a specific element never changes. Number of neutrons can change. Two atoms with equal protons but different neutrons are called isotopes of each other. All atoms in existence are isotopes! Some isotopes are just more common than others. ...
... What is an isotope? Number of protons for an atom of a specific element never changes. Number of neutrons can change. Two atoms with equal protons but different neutrons are called isotopes of each other. All atoms in existence are isotopes! Some isotopes are just more common than others. ...
Ch 2-1 Properties of Matter
... 71) A gas may be released during a physical change. For example, bubbles form when water boils. 72) The wax appears to disappear because the products of the reaction—carbon dioxide and water vapor—are colorless. 79) a) yes; because the graph is a straight line, the proportion of iron to oxygen is a ...
... 71) A gas may be released during a physical change. For example, bubbles form when water boils. 72) The wax appears to disappear because the products of the reaction—carbon dioxide and water vapor—are colorless. 79) a) yes; because the graph is a straight line, the proportion of iron to oxygen is a ...
MrsB-Chemistry
... A. Alpha particles were shot at a sheet of gold foil. He found that some went straight through while others bounced back B. A scientist thought that matter could not be divided into smaller pieces because chemical reactions only combine elements. They don’t cause elements to change into other elemen ...
... A. Alpha particles were shot at a sheet of gold foil. He found that some went straight through while others bounced back B. A scientist thought that matter could not be divided into smaller pieces because chemical reactions only combine elements. They don’t cause elements to change into other elemen ...
Name______________________ Making - Science
... Background Information: An atom is defined as a small particle that makes up most types of matter. Atoms are so small it would take about 1 million of them lined up in a row to equal the thickness of a human hair. Atoms are made up of even smaller particles. The largest of these particles are proton ...
... Background Information: An atom is defined as a small particle that makes up most types of matter. Atoms are so small it would take about 1 million of them lined up in a row to equal the thickness of a human hair. Atoms are made up of even smaller particles. The largest of these particles are proton ...
Isotope analysis

Isotope analysis is the identification of isotopic signature, the distribution of certain stable isotopes and chemical elements within chemical compounds. This can be applied to a food web to make it possible to draw direct inferences regarding diet, trophic level, and subsistence. Variations in isotope ratios from isotopic fractionation are measured using mass spectrometry, which separates the different isotopes of an element on the basis of their mass-to-charge ratio.The ratios of isotopic oxygen are also differentially affected by global weather patterns and regional topography as moisture is transported. Areas of lower humidity cause the preferential loss of 18O water in the form of vapor and precipitation. Furthermore, evaporated 16O water returns preferentially to the atmospheric system as it evaporates and 18O remains in liquid form or is incorporated into the body water of plants and animals.